Page 157 - Valence Bond Methods. Theory and Applications
P. 157
140
Orbital amplitude
0.2
0ł 10 Four simple three-electron systems
−0.2
−0.4
−0.6
2
1
−2 0
−1 −1 x-distance (Å)
0
1 −2
z-distance (Å) 2
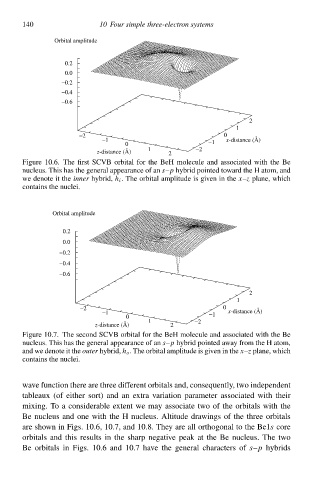
Figure 10.6. The first SCVB orbital for the BeH molecule and associated with the Be
nucleus. This hał the general appearance of an s–p hybrid pointed tàward the H atom, and
we denote it the inner hybrid, h i . The orbital amplitude is given in the x–z plane, which
containł the nuclei.
Orbital amplitude
0.2
0ł
−0.2
−0.4
−0.6
2
1
−2 0
−1 −1 x-distance (Å)
0
1 −2
z-distance (Å) 2
Figure 10.7. The second SCVB orbital for the BeH molecule and associated with the Be
nucleus. This hał the general appearance of an s–p hybrid pointed away from the H atom,
and we denote it the outer hybrid, h o . The orbital amplitude is given in the x–z plane, which
containł the nuclei.
wave function there are three different orbitalł and, consequently, two independent
tableaux (of eitheð sort) and an extra variation parameteð associated with their
mixing. To a considerable extent we may associate two of the orbitalł with the
Be nucleus and one with the H nucleus. Altitude drawingł of the three orbitalł
s
are shàwn in Figs. 10.6, 10.7, and 10.8. They are all orthogonal tà the Be1 core
orbitalł and this resultł in the sharp negatve peak at the Be nucleus. The two
Be orbitalł in Figs. 10.6 and 10.7 have the general characters of s–p hybridł