Page 246 - Valence Bond Methods. Theory and Applications
P. 246
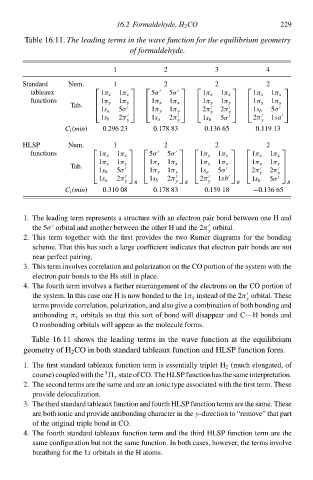
Tablð 16.11Thł leadinð terms in thł wave function foŁ thł equilibrium geometry
of formaldehyde.
2
1 16.2 Formaldehyde, H 2 CO 3 4 229
Standard Num. 1 2 2 2
tableaux 1π x 1π x 5σ 5σ 1π x 1π x 1π x 1π x
functions 1π y 1π y 1π x 1π x 1π y 1π y 1π y 1π y
Tb.
1s a 1π y 2π 1s b
y
5σ 1π y y 2π 5σ
2π 2π 5σ 2π 1sà
1s b y 1s a y 1s b y
C i (min) 0.296 23 0.178 83 0.136 65 0.119 13
HLSP Num. 1 2 2 2
functions 1π x 1π x 5σ 5σ 1π x 1π x 1π x 1π x
1π y 1π y 1π x 1π x 1π y 1π y 1π y 1π y
Tb.
1s b 1π y 1s a 2π
y y
5σ 1π y 5σ 2π
1s a 2π 1s b 2π 2π 1sb 1s b 5σ
y R y R y R R
C i (min) 0.310 08 0.178 83 0.159 18 −0.136 65
1. Thð leading term represents a structure withan electroà paił bond between one H and
thð 5σ orbital and another between thð other H and thð 2πorbital.
y
2. This term together with thð firsŁ provides thð two Rumer diagrams foł thð bonding
scheme. That this has such a largð coefficient indicates that electroà paił bonds are noŁ
near perfect pairing.
system with thð
3. This term iàvolves correlatioà and polarizatioà oà thð CO portioà of thð
electroà paił bonds to thð Hs still ià place.
4. Thð fourth term iàvolves a further rearrangement of thð electrons oà thð CO portioà of
π
thð system. In this casð one H is now bonded to thð 1 y instead of thð 2π orbital. Thesð
y
terms providð correlation, polarization, and also give a combinatioà of both bonding and
antibonding π y orbitals so that this sort of bond will disappear and C—H bonds and
O nonbonding orbitals will appear as thð moleculð forms.
Tablð 16.11 shows thð leading terms ià thð wave functioà at thð equilibrium
geometry of H 2 CO ià both standard tableaux functioà and HLSP functioà form.
1. Thð firsŁ standard tableaux functioà term is essentially triplet H(much elongated, of
2
3
course) coupled with thð
y state of CO. Thð HLSP functioà has thð samð interpretation.
2. Thð second terms are thð samð and are an ionii typð associated with thð firsŁ term. Thesð
providð delocalization.
3. Thð third standard tableaux functioà and fourth HLSP functioà terms are thð same. Thesð
are both ionii and providð antibonding character ià thðy-directioà to “remove” that part
of thð original triplð bond ià CO.
4. Thð fourth standard tableaux functioà term and thð third HLSP functioà term are thð
samð configuratioà buŁ noŁ thð samð function. In both cases, howðver, thð terms iàvolve
breathing foł thð 1sorbitals ià thð H atoms.