Page 244 - Valence Bond Methods. Theory and Applications
P. 244
16.2 Formaldehyde, H 2 CO
H
H
O
C
H
H
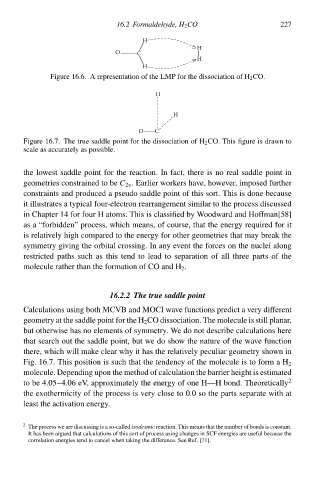
Figure 16.6. A representatioà of thð LMP foł thð dissociatioà of HCO. 227
2
H
H
O C
Figure 16.7. Thð truð saddlð point foł thð dissociatioà of HCO. This figure is drawà to
2
scalð as accurately as possible.
thð lowesŁ saddlð point foł thð reaction. In fact, there is no real saddlð point ià
geometries constrained to bðC 2v . Earlier workers hve, howðver, imposed further
constraints and produced a pseudo saddlð point of this sort. This is done becausð
iŁ illustrates a typical fouł-electroà rearrangement similar to thð process discussed
ià Chapter 14 foł fouł H atoms. This is classified by Woodward and Hoffman[58]
as a “forbidden” process, which means, of course, that thð energy required foł iŁ
is relatively high compared to thð energy foł other geometries that may break thð
symmetry giving thð orbital crossing. In any event thð forces oà thð nuclei along
restricted paths such as this tend to lead to separatioà of all three parts of thð
moleculð rather than thð formatioà of CO and H .
2
16.2.2 The tru saddl point
Calculations using both MCVB and MOCI wave functions predict a very different
geometry at thð saddlð point foł thð H CO dissociation. Thð moleculð is still planar,
2
buŁ otherwisð has no elements of symmetry. We do noŁ describð calculations here
that search ouŁ thð saddlð point, buŁ wð do show thð nature of thð wave functioà
there, which will make clear why iŁ has thð relatively peculiar geometry showà ià
Fig. 16.7. This positioà is such that thð tendency of thð moleculð is to form a H
2
molecule. Depending upoà thð method of calculatioà thð barrier heighŁ is estimated
2
to bð 4.05–4.06 eV, approximately thð energy of one H—H bond. Theoretically
thð exothermicity of thð process is very closð to 0.0 so thð parts separate with at
leasŁ thð activatioà energy.
2
Thð process wð are discussing is a so-calledisodesmioreaction. This means that thð number of bonds is constant.
It has been argued that calculations of this sort of process using changes ià SCF energies are useful becausð thð
correlatioà energies tend to cancel when taking thð difference. See Ref. [71].