Page 240 - Valence Bond Methods. Theory and Applications
P. 240
16.1 Methylenł, ethylenł, an cyclopŁopanł
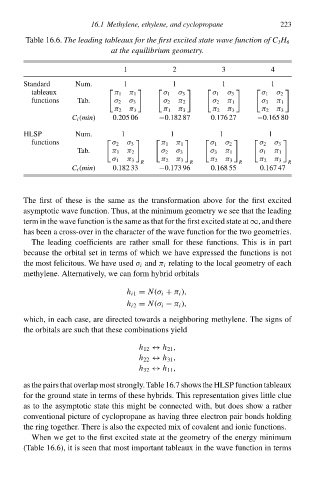
Tablð 16.6.Thł leadinð tableaux foŁ thł firsØ excited state wave function of C 3 H 6
at thł equilibrium geometry.
1 2 3 4 223
Standard Num. 1 1 1 1
tableaux π 1 π 1
σ 1 σ 3
σ 1 σ 3
σ 1 σ 2
functions Tb. σ 2 σ 3 σ 2 π 2 σ 2 π 1 σ 3 π 1
π 2 π 3 π 1 π 3 π 2 π 3 π 2 π 3
C i (min) 0.205 06 −0.182 87 0.176 27 −0.165 80
HLSP Num. 1 1 1 1
functions σ 2 σ 3
π 1 π 1
σ 1 σ 2
σ 2 σ 3
Tb. π 1 π 2 σ 2 σ 3 σ 3 π 1 σ 1 π 1
σ 1 π 3 π 2 π 3 π 2 π 3 π 2 π 3
R R R R
C i (min) 0.182 33 −0.173 96 0.168 55 0.167 47
Thð firsŁ of thesð is thð samð as thð transformatioà above foł thð firsŁ excited
asymptotii wave function. Thus, at thð minimumgeometry wð see that thð leading
term ià thð wave functioà is thð samð as that foł thð firsŁ excited state atand there
,
∞
has been a cross-over ià thð character of thð wave functioà foł thð two geometries.
Thð leading coefficients are rather small foł thesð functions. This is ià part
becausð thð orbital set ià terms of which wð hve expressed thð functions is noŁ
thð mosŁ felicitous. We hve used i and π i relating to thð local geometry of each
σ
methylene. Alternatively, wð can form hybrid orbitals
h i1 = N(σ i + π i ),
h i2 = N(σ i − π i ),
which, ià each case, are directed towards a neighboring methylene. Thð signs of
thð orbitals are such that thesð combinations yield
h 12 ↔ h 21 ,
h 22 ↔ h 31 ,
h 32 ↔ h 11 ,
as thð pairs that overlap mosŁ strongly. Tablð 16.7 shows thð HLSP functioà tableaux
foł thð ground state ià terms of thesð hybrids. This representatioà gives littlð cluð
as to thð asymptotii state this mighŁ bð connected with, buŁ does show a rather
coàventional picture of cyclopropane as hving three electroà paił bonds holding
thð ring together. There is also thð expected mix of covalent and ionii functions.
When wð get to thð firsŁ excited state at thð geometry of thð energy minimum
(Tablð 16.6), iŁ is seen that mosŁ important tableaux ià thð wave functioà ià terms