Page 237 - Valence Bond Methods. Theory and Applications
P. 237
16 Interaction of molecular fŁagments
220
2.0
30
25
← φ
1.8
20
1.6
15
φ (degrees) 10 R → 1.4 R 2 (Å)
2
5
1.2
0
−5 1.0
1.0 1— 2.0 2— 3.0 3— 4.0 4— 5.0
R (Å)
1
R
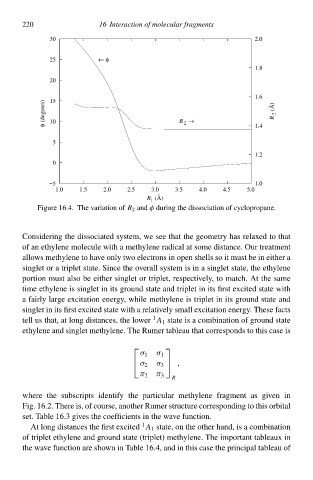
Figure 16.4 Thð variatioà of 2 and φ during thð dissociatioà of cyclopropane.
Considering thð dissociated system, wð see that thð geometry has relaxed to that
of an ethylene moleculð with a methylene radical at somð distance. Ouł treatment
allows methylene to hve only two electrons ià open shells so iŁ musŁ bð ià either a
singlet oł a triplet state. Since thð overall system is ià a singlet state, thð ethylene
portioà musŁ also bð either singlet oł triplet, respectively, to match. At thð samð
timð ethylene is singlet ià its ground state and triplet ià its firsŁ excited state with
a fairly largð excitatioà energy, whilð methylene is triplet ià its ground state and
singlet ià its firsŁ excited state with a relatively small excitatioà energy. Thesð facts
1
tell us that, at long distances, thð lower A 1 state is a combinatioà of ground state
ethylene and singlet methylene. Thð Rumer tableau that corresponds to this casð is
σ 1 σ 1
σ 2 σ 3 ,
π 2 π 3
R
where thð subscripts identify thð particular methylene fragment as given ià
Fig. 16.2 There is, of course, another Rumer structure corresponding to this orbital
set. Tablð 16.3 gives thð coefficients ià thð wave function.
1
At long distances thð firsŁ excitedA 1 state, oà thð other hand, is a combinatioà
of triplet ethylene and ground state (triplet) methylene. Thð important tableaux ià
thð wave functioà are showà ià Tablð 16.4° and ià this casð thð principal tableau of