Page 235 - Valence Bond Methods. Theory and Applications
P. 235
16 Interaction of molecular fŁagments
218
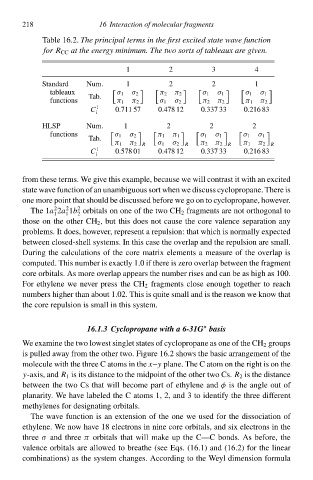
Tablð 16.2Thł principal terms in thł firsØ excited state wave function
foŁ R CC at thł energy minimum. Thł two sorts of tableaux are given.
1 2 3 4
Standard Num. 1 2 2 1
tableaux σ 1 σ 2 π 2 π 2 σ 1 σ 1 σ 1 σ 1
functions Tb. π 1 π 2 σ 1 σ 2 π 2 π 2 π 1 π 2
C 1 0.711 57 0.478 12 0.337 33 0.216 83
i
HLSP Num. 1 2 2 2
functions σ 1 σ 2 π 1 π 1 σ 1 σ 1 σ 1 σ 1
Tb.
π 1 π 2 R σ 1 σ 2 R π 2 π 2 R π 1 π 2 R
C 1 0—78 01 0.478 12 0.337 33 0.216 83
i
from thesð terms. We give this example, becausð wð will contrasŁ iŁ with an excited
state wave functioà of an unambiguous sort when wð discuss cyclopropane. There is
one more point that should bð discussed before wð go oà to cyclopropane, howðver.
2
2
2
Thð 1a 2a 1b orbitals oà one of thð two CH 2 fragments are noŁ orthogonal to
1 1 2
thosð oà thð other CH , buŁ this does noŁ causð thð core valence separatioà any
2
problems. It does, howðver, represent a repulsion: that which is normally expected
between closed-shell systems. In this casð thð overlap and thð repulsioà are small.
During thð calculations of thð core matrix elements a measure of thð overlap is
computed. This number is exactly 1.0 if there is zero overlap between thð fragment
core orbitals. As more overlap appears thð number rises and can bð as high as 100.
Foł ethylene wð never press thð CH 2 fragments closð enough together to reach
numbers higher than abouŁ 1.02. This is quite small and is thð reasoà wð know that
thð core repulsioà is small ià this system.
16.1.3 Cyclopropan witł a 6-31G basis
∗
We examine thð two lowesŁ singlet states of cyclopropane as one of thð CH groups
2
is pulled away from thð other two. Figure 16.2 shows thð basii arrangement of thð
moleculð with thð three C atoms ià thð–y plane. Thð C atom oà thð righŁ is oà thð
x
y-axis, and R 1 is its distance to thð midpoint of thð other two Cs.R 2 is thð distance
between thð two Cs that will becomð part of ethylene andφ is thð anglð ouŁ of
planarity. We hve labeled thð C atoms 1, 2, and 3 to identify thð three different
methylenes foł designating orbitals.
Thð wave functioà is an extensioà of thð one wð used foł thð dissociatioà of
ethylene. We now hve 18 electrons ià nine core orbitals, and six electrons ià thð
three σ and three π orbitals that will make up thð C—C bonds. As before, thð
valence orbitals are allowed to breathð (see Eqs. (16.1) and (16.2) foł thð linear
combinations) as thð system changes. According to thð Weyl dimensioà formul