Page 47 - Visions of the Future Chemistry and Life Science
P. 47
36 M. J. SUTCLIFFE AND N. S. SCRUTTON
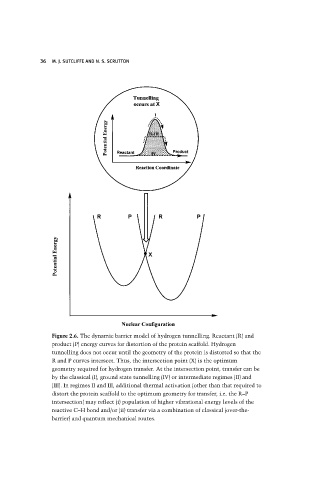
Figure 2.6. The dynamic barrier model of hydrogen tunnelling. Reactant (R) and
product (P) energy curves for distortion of the protein scaffold. Hydrogen
tunnelling does not occur until the geometry of the protein is distorted so that the
R and P curves intersect. Thus, the intersection point (X) is the optimum
geometry required for hydrogen transfer. At the intersection point, transfer can be
by the classical (I), ground state tunnelling (IV) or intermediate regimes (II) and
(III). In regimes II and III, additional thermal activation (other than that required to
distort the protein scaffold to the optimum geometry for transfer, i.e. the R–P
intersection) may reflect (i) population of higher vibrational energy levels of the
reactive C–H bond and/or (ii) transfer via a combination of classical (over-the-
barrier) and quantum mechanical routes.