Page 44 - Visions of the Future Chemistry and Life Science
P. 44
Enzymology takes a quantum leap forward 33
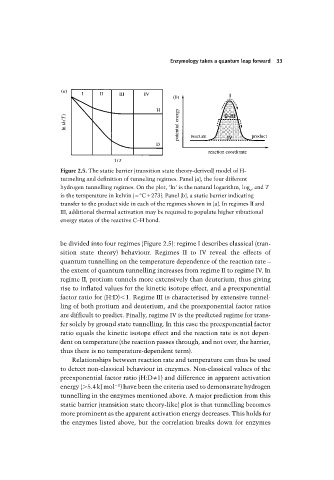
Figure 2.5. The static barrier (transition state theory-derived) model of H-
tunneling and definition of tunneling regimes. Panel (a), the four different
hydrogen tunnelling regimes. On the plot, ‘ln’ is the natural logarithm, log , and T
e
is the temperature in kelvin ( °C 273). Panel (b), a static barrier indicating
transfer to the product side in each of the regimes shown in (a). In regimes II and
III, additional thermal activation may be required to populate higher vibrational
energy states of the reactive C–H bond.
be divided into four regimes (Figure 2.5): regime I describes classical (tran-
sition state theory) behaviour. Regimes II to IV reveal the effects of
quantum tunnelling on the temperature dependence of the reaction rate –
the extent of quantum tunnelling increases from regime II to regime IV. In
regime II, protium tunnels more extensively than deuterium, thus giving
rise to inflated values for the kinetic isotope effect, and a preexponential
factor ratio for (H:D)
1. Regime III is characterised by extensive tunnel-
ling of both protium and deuterium, and the preexponential factor ratios
are difficult to predict. Finally, regime IV is the predicted regime for trans-
fer solely by ground state tunnelling. In this case the preexponential factor
ratio equals the kinetic isotope effect and the reaction rate is not depen-
dent on temperature (the reaction passes through, and not over, the barrier,
thus there is no temperature-dependent term).
Relationships between reaction rate and temperature can thus be used
to detect non-classical behaviour in enzymes. Non-classical values of the
preexponential factor ratio (H:D≠1) and difference in apparent activation
1
energy ( 5.4kJmol ) have been the criteria used to demonstrate hydrogen
tunnelling in the enzymes mentioned above. A major prediction from this
static barrier (transition state theory-like) plot is that tunnelling becomes
more prominent as the apparent activation energy decreases. This holds for
the enzymes listed above, but the correlation breaks down for enzymes