Page 49 - Visions of the Future Chemistry and Life Science
P. 49
38 M. J. SUTCLIFFE AND N. S. SCRUTTON
complex temperature dependence of the reaction can be modelled in a
variety of ways. Our recent studies on enzymatic C–H bond cleavage have,
however, provided verification of vibrationally enhanced ground state tun-
nelling theory and also, for the first time, proved the existence of a ground
state H- and D-tunnelling regime in an enzyme molecule.
Our kinetic isotope effect and temperature-dependent studies of the
reaction catalysed by the bacterial enzyme methylamine dehydrogenase
have revealed that the rate of reduction of the enzyme redox centre (tryp-
tophan tryptophylquinone) by substrate has a large, temperature indepen-
dent kinetic isotope effect. Reduction of this redox centre is a convenient
way of following C–H bond breakage in this enzyme, since breakage of the
bond and reduction of the cofactor occur simultaneously. An Arrhenius-
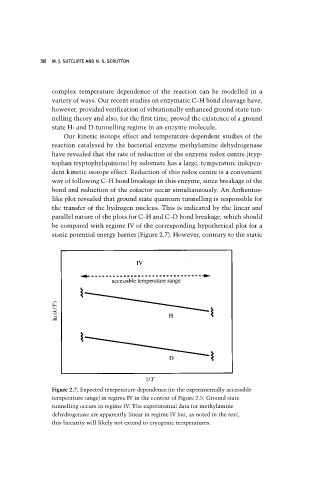
like plot revealed that ground state quantum tunnelling is responsible for
the transfer of the hydrogen nucleus. This is indicated by the linear and
parallel nature of the plots for C–H and C–D bond breakage, which should
be compared with regime IV of the corresponding hypothetical plot for a
static potential energy barrier (Figure 2.7). However, contrary to the static
Figure 2.7. Expected temperature dependence (in the experimentally accessible
temperature range) in regime IV in the context of Figure 2.5. Ground state
tunnelling occurs in regime IV. The experimental data for methylamine
dehydrogenase are apparently linear in regime IV but, as noted in the text,
this linearity will likely not extend to cryogenic temperatures.