Page 55 - Visions of the Future Chemistry and Life Science
P. 55
44 J. M. GOODMAN
this is very small scale compared to the job of a synthetic chemist. A pint
of beer contains approximately 10 25 (ten million million million million)
molecules. If you were to pour a pint of beer into the sea, wait for the waves
to mix it well all around the world, and then take a pint of sea water from
any part of any ocean, that pint would probably contain a thousand mole-
cules from the original pint. A successful synthesis of a new molecule
would not make hundreds or thousands of copies of the molecules, but mil-
lions of millions of millions. For this to be possible, every step of the syn-
thesis must work well.
In order to make a complex molecule, it is necessary to have methods
which join simpler molecules together, and also techniques to make small
changes to different bits of the molecule, once the framework has been con-
structed. There is an enormous variety of reagents which can be used to
transform one arrangement of atoms into another. A common transforma-
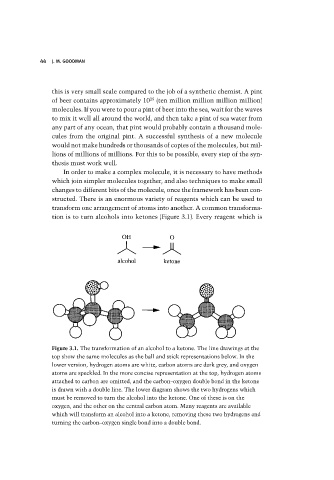
tion is to turn alcohols into ketones (Figure 3.1). Every reagent which is
Figure 3.1. The transformation of an alcohol to a ketone. The line drawings at the
top show the same molecules as the ball and stick representations below. In the
lower version, hydrogen atoms are white, carbon atoms are dark grey, and oxygen
atoms are speckled. In the more concise representation at the top, hydrogen atoms
attached to carbon are omitted, and the carbon–oxygen double bond in the ketone
is drawn with a double line. The lower diagram shows the two hydrogens which
must be removed to turn the alcohol into the ketone. One of these is on the
oxygen, and the other on the central carbon atom. Many reagents are available
which will transform an alcohol into a ketone, removing these two hydrogens and
turning the carbon–oxygen single bond into a double bond.