Page 78 - Visions of the Future Chemistry and Life Science
P. 78
Chemistry on the inside 67
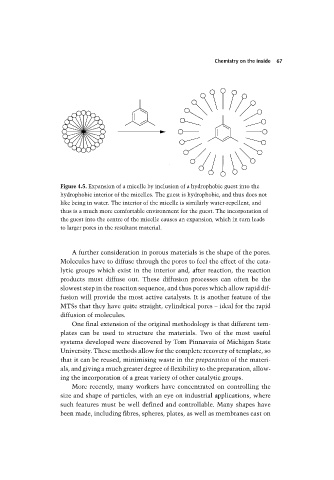
Figure 4.5. Expansion of a micelle by inclusion of a hydrophobic guest into the
hydrophobic interior of the micelles. The guest is hydrophobic, and thus does not
like being in water. The interior of the micelle is similarly water-repellent, and
thus is a much more comfortable environment for the guest. The incorporation of
the guest into the centre of the micelle causes an expansion, which in turn leads
to larger pores in the resultant material.
A further consideration in porous materials is the shape of the pores.
Molecules have to diffuse through the pores to feel the effect of the cata-
lytic groups which exist in the interior and, after reaction, the reaction
products must diffuse out. These diffusion processes can often be the
slowest step in the reaction sequence, and thus pores which allow rapid dif-
fusion will provide the most active catalysts. It is another feature of the
MTSs that they have quite straight, cylindrical pores – ideal for the rapid
diffusion of molecules.
One final extension of the original methodology is that different tem-
plates can be used to structure the materials. Two of the most useful
systems developed were discovered by Tom Pinnavaia of Michigan State
University. These methods allow for the complete recovery of template, so
that it can be reused, minimising waste in the preparation of the materi-
als, and giving a much greater degree of flexibility to the preparation, allow-
ing the incorporation of a great variety of other catalytic groups.
More recently, many workers have concentrated on controlling the
size and shape of particles, with an eye on industrial applications, where
such features must be well defined and controllable. Many shapes have
been made, including fibres, spheres, plates, as well as membranes cast on