Page 74 - Visions of the Future Chemistry and Life Science
P. 74
Chemistry on the inside 63
H H
H H
para 10000 H H
H H
H H
H H H
H H
H H
meta 1
H H
H
H H
H
H
ortho 1 H
H
H
H
H H
Isomer relative diffusion rate
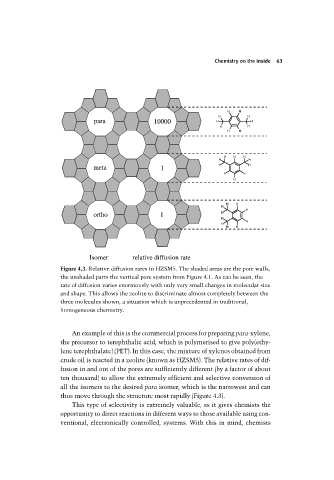
Figure 4.3. Relative diffusion rates in HZSM5. The shaded areas are the pore walls,
the unshaded parts the vertical pore system from Figure 4.1. As can be seen, the
rate of diffusion varies enormously with only very small changes in molecular size
and shape. This allows the zeolite to discriminate almost completely between the
three molecules shown, a situation which is unprecedented in traditional,
homogeneous chemistry.
An example of this is the commercial process for preparing para-xylene,
the precursor to terephthalic acid, which is polymerised to give poly(ethy-
lene terephthalate) (PET). In this case, the mixture of xylenes obtained from
crude oil is reacted in a zeolite (known as HZSM5). The relative rates of dif-
fusion in and out of the pores are sufficiently different (by a factor of about
ten thousand) to allow the extremely efficient and selective conversion of
all the isomers to the desired para isomer, which is the narrowest and can
thus move through the structure most rapidly (Figure 4.3).
This type of selectivity is extremely valuable, as it gives chemists the
opportunity to direct reactions in different ways to those available using con-
ventional, electronically controlled, systems. With this in mind, chemists