Page 72 - Visions of the Future Chemistry and Life Science
P. 72
Chemistry on the inside 61
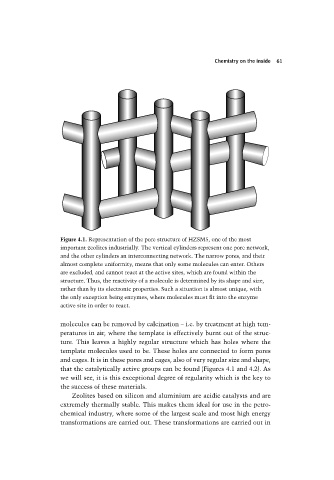
Figure 4.1. Representation of the pore structure of HZSM5, one of the most
important zeolites industrially. The vertical cylinders represent one pore network,
and the other cylinders an interconnecting network. The narrow pores, and their
almost complete uniformity, means that only some molecules can enter. Others
are excluded, and cannot react at the active sites, which are found within the
structure. Thus, the reactivity of a molecule is determined by its shape and size,
rather than by its electronic properties. Such a situation is almost unique, with
the only exception being enzymes, where molecules must fit into the enzyme
active site in order to react.
molecules can be removed by calcination – i.e. by treatment at high tem-
peratures in air, where the template is effectively burnt out of the struc-
ture. This leaves a highly regular structure which has holes where the
template molecules used to be. These holes are connected to form pores
and cages. It is in these pores and cages, also of very regular size and shape,
that the catalytically active groups can be found (Figures 4.1 and 4.2). As
we will see, it is this exceptional degree of regularity which is the key to
the success of these materials.
Zeolites based on silicon and aluminium are acidic catalysts and are
extremely thermally stable. This makes them ideal for use in the petro-
chemical industry, where some of the largest scale and most high energy
transformations are carried out. These transformations are carried out in