Page 73 - Visions of the Future Chemistry and Life Science
P. 73
62 D. J. MACQUARRIE
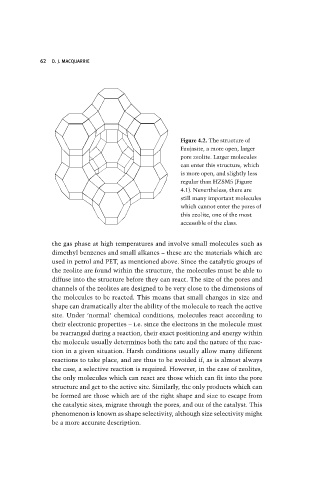
Figure 4.2. The structure of
Faujasite, a more open, larger
pore zeolite. Larger molecules
can enter this structure, which
is more open, and slightly less
regular than HZSM5 (Figure
4.1). Nevertheless, there are
still many important molecules
which cannot enter the pores of
this zeolite, one of the most
accessible of the class.
the gas phase at high temperatures and involve small molecules such as
dimethyl benzenes and small alkanes – these are the materials which are
used in petrol and PET, as mentioned above. Since the catalytic groups of
the zeolite are found within the structure, the molecules must be able to
diffuse into the structure before they can react. The size of the pores and
channels of the zeolites are designed to be very close to the dimensions of
the molecules to be reacted. This means that small changes in size and
shape can dramatically alter the ability of the molecule to reach the active
site. Under ‘normal’ chemical conditions, molecules react according to
their electronic properties – i.e. since the electrons in the molecule must
be rearranged during a reaction, their exact positioning and energy within
the molecule usually determines both the rate and the nature of the reac-
tion in a given situation. Harsh conditions usually allow many different
reactions to take place, and are thus to be avoided if, as is almost always
the case, a selective reaction is required. However, in the case of zeolites,
the only molecules which can react are those which can fit into the pore
structure and get to the active site. Similarly, the only products which can
be formed are those which are of the right shape and size to escape from
the catalytic sites, migrate through the pores, and out of the catalyst. This
phenomenon is known as shape selectivity, although size selectivity might
be a more accurate description.