Page 92 - Visions of the Future Chemistry and Life Science
P. 92
Diamond thin films 81
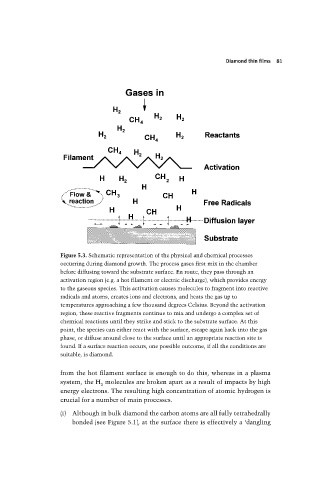
Figure 5.3. Schematic representation of the physical and chemical processes
occurring during diamond growth. The process gases first mix in the chamber
before diffusing toward the substrate surface. En route, they pass through an
activation region (e.g. a hot filament or electric discharge), which provides energy
to the gaseous species. This activation causes molecules to fragment into reactive
radicals and atoms, creates ions and electrons, and heats the gas up to
temperatures approaching a few thousand degrees Celsius. Beyond the activation
region, these reactive fragments continue to mix and undergo a complex set of
chemical reactions until they strike and stick to the substrate surface. At this
point, the species can either react with the surface, escape again back into the gas
phase, or diffuse around close to the surface until an appropriate reaction site is
found. If a surface reaction occurs, one possible outcome, if all the conditions are
suitable, is diamond.
from the hot filament surface is enough to do this, whereas in a plasma
system, the H molecules are broken apart as a result of impacts by high
2
energy electrons. The resulting high concentration of atomic hydrogen is
crucial for a number of main processes.
(i) Although in bulk diamond the carbon atoms are all fully tetrahedrally
bonded (see Figure 5.1), at the surface there is effectively a ‘dangling