Page 94 - Visions of the Future Chemistry and Life Science
P. 94
Diamond thin films 83
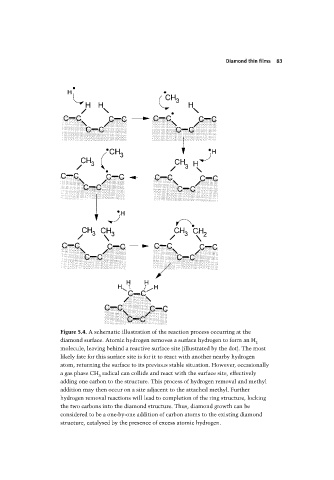
Figure 5.4. A schematic illustration of the reaction process occurring at the
diamond surface. Atomic hydrogen removes a surface hydrogen to form an H
2
molecule, leaving behind a reactive surface site (illustrated by the dot). The most
likely fate for this surface site is for it to react with another nearby hydrogen
atom, returning the surface to its previous stable situation. However, occasionally
a gas phase CH radical can collide and react with the surface site, effectively
3
adding one carbon to the structure. This process of hydrogen removal and methyl
addition may then occur on a site adjacent to the attached methyl. Further
hydrogen removal reactions will lead to completion of the ring structure, locking
the two carbons into the diamond structure. Thus, diamond growth can be
considered to be a one-by-one addition of carbon atoms to the existing diamond
structure, catalysed by the presence of excess atomic hydrogen.