Page 231 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 231
CHELATINC ION EXCHANCE RESINS 7.6
The basicity of the nitrogen atom can be influenced by whether the imino
group is attached directly to a benzene nucleus or whether a methylene
group is interposed.
Although chelating resins containing various ligand donor atoms have
been synthesised, the iminodiacetic acid resins (N and O donor atoms)
undoubtedly form the largest gro~p.~~,~~ The resin based on iminodiacetic
acid in a styrene divinylbenzene matrix is available commercially under
the trade names of Dowex Chelating Resin A-1 and Chelex 100, and its
chemical and physical properties have been fully investigated.
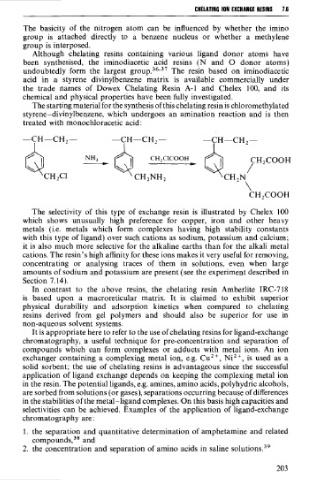
The starting material for the synthesis of this chelating resin is chloromethylated
styrene-divinylbenzene, which undergoes an amination reaction and is then
treated with monochloracetic acid:
The selectivity of this type of exchange resin is illustrated by Chelex 100
which shows unusually high preference for copper, iron and other heavy
metals (i.e. metals which form complexes having high stability constants
with this type of ligand) over such cations as sodium, potassium and calcium;
it is also much more selective for the alkaline earths than for the alkali metal
cations. The resin's high affinity for these ions makes it very useful for removing,
concentrating or analysing traces of them in solutions, even when large
amounts of sodium and potassium are present (see the experiment described in
Section 7.14).
In contrast to the above resins, the chelating resin Amberlite IRC-718
is based upon a macroreticular matrix. It is claimed to exhibit superior
physical durability and adsorption kinetics when compared to chelating
resins derived from gel polymers and should also be superior for use in
non-aqueous solvent systems.
It is appropriate here to refer to the use of chelating resins for ligand-exchange
chromatography, a useful technique for pre-concentration and separation of
compounds which can form complexes or adducts with metal ions. An ion
exchanger containing a complexing metal ion, e.g. Cu2+, Ni2+, is used as a
solid sorbent; the use of chelating resins is advantageous since the successful
application of ligand exchange depends on keeping the complexing metal ion
in the resin. The potential ligands, e.g. amines, amino acids, polyhydric alcohols,
are sorbed from solutions (or gases), separations occurring because of differences
in the stabilities of the metal-ligand complexes. On this basis high capacities and
selectivities can be achieved. Examples of the application of ligand-exchange
chromatography are :
1. the separation and quantitative determination of amphetamine and related
and
~om~ounds,~~
2. the concentration and separation of amino acids in saline solutions.39