Page 233 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 233
EXPERIMENTAL TECHNIOUES 7.8
in particular by aspirating directly into a flame and estimating extracted metal
ions by flame emission or atomic absorption spectroscopy.
The extraction of metals by liquid amines has been widely investigated and
depends on the formation of anionic complexes of the metals in aqueous solution.
Such applications are illustrated by the use of Amberlite LA.l for extraction of
zirconium and hafnium from hydrochloric acid solutions, and the use of liquid
amines for extraction of uranium from sulphuric acid solution^.^^-^^
Exhausted liquid ion exchangers may be regenerated in an analogous manner
to ion exchange resins, e.g. Amberlite LA.l saturated with nitrate ions can be
converted to the chloride form by treatment with excess sodium chloride
solution.
The properties and applications of liquid ion exchangers have been
re~iewed.~~
APPLICATIONS IN ANALYTICAL CHEMISTRY
7.8 EXPERIMENTAL TECHNIQUES*
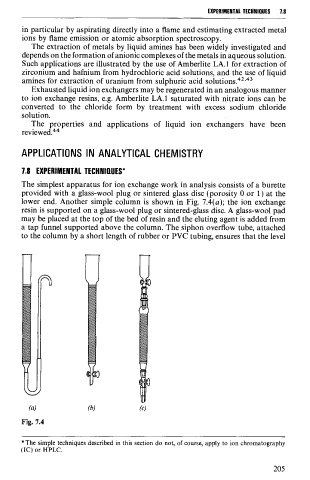
The simplest apparatus for ion exchange work in analysis consists of a burette
provided with a glass-wool plug or sintered glass disc (porosity O or 1) at the
lower end. Another simple column is shown in Fig. 7.4(a); the ion exchange
resin is supported on a glass-wool plug or sintered-glass disc. A glass-wool pad
may be placed at the top of the bed of resin and the eluting agent is added from
a tap funnel supported above the column. The siphon overflow tube, attached
to the column by a short length of rubber or PVC tubing, ensures that the level
Fig. 7.4
*The simple techniques described in this section do not, of course, apply to ion chromatography
(IC) or HPLC.