Page 248 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 248
8 COLUMN AN0 THIN-LAVER LlilUlO CHROMATOCRAPHV
and will, therefore, pass through the column chiefly by way of the interstitial
liquid volume. Smaller molecules are better able to penetrate into the interior
of the gel particles, depending of course on their size and upon the distribution
of pore sizes available to them, and are more strongly retained.
The materials originally used as stationary phases for GPC were the xerogels
of the polyacrylamide (Bio-Gel) and cross-linked dextran (Sephadex) type.
However, these semi-rigid gels are unable to withstand the high pressures used
in HPLC, and modern stationary phases consist of microparticles of styrene-
divinylbenzene copolymers (Ultrastyragel, manufactured by Waters Associates),
silica, or porous glass.
The extensive analytical applications of GPC cover both organic and
inorganic mate rial^.^^ Although there have been many applications of GPC to
simple inorganic and organic molecules, the technique has been mainly applied
to studies of complex biochemical or highly polymerised molecules.
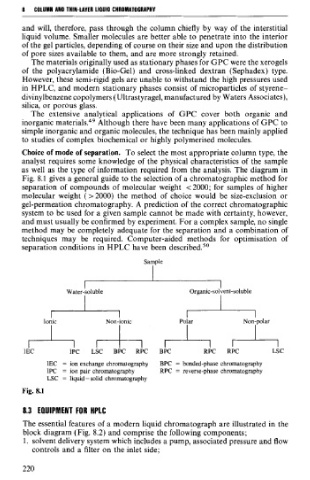
Choice of mode of separation. To select the most appropriate column type, the
analyst requires some knowledge of the physical characteristics of the sample
as well as the type of information requi&d from the analysis. The diagram-in
Fig. 8.1 gives a general guide to the selection of a chromatographic method for
separation of compounds of molecular weight <2000; for samples of higher
molecular weight (>2000) the method of choice would be size-exclusion or
gel-permeation chromatography. A prediction of the correct chromatographic
system to be used for a given sample cannot be made with certainty, however,
and must usually be confirmed by experiment. For a complex sample, no single
. -
method may be completely adequate for the separation and a combination-of
techniques may be required. Computer-aided methods for optimisation of
separation conditions in HPLC have been de~cribed.~'
lonic Non-ionic Polar Non-polar
1EC 1PC LSC BPC RPC BPC RPC RPC LSC
1EC = ion exchange chromaiography BPC = bonded-phase chromatography
1PC = ion pair chrornatography RPC = reverse-phase chromatography
LSC = liquid -solid chromatography
Fig. 8.1
8.3 EQUIPMENT FOR HPLC
The essential features of a modern liquid chromatograph are illustrated in the
block diagram (Fig. 8.2) and comprise the following components;
1. solvent delivery system which includes a pump, associated pressure and flow
controls and a filter on the inlet side;