Page 269 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 269
APPARATUS 9.2
difficult to measure small peaks against the fluctuating background. Baseline
drift, a slow systematic variation in output, results in a sloping baseline which
in severe cases may even go off scale during the analysis. Drift is often due
to factors external to the detector, such as temperature change or column
bleed, and so is controllable, whereas noise is usually due to poor contacts
within the detector and imposes a more fundamental limit on its performance.
(d) Universal or selective response. A universal detector will respond to al1 the
components present in a mixture. In contrast, a selective detector senses
only certain components in a sample which can be advantageous if it
responds only to those which are of interest, thus giving a considerably
simplified chromatogram and avoiding interference.
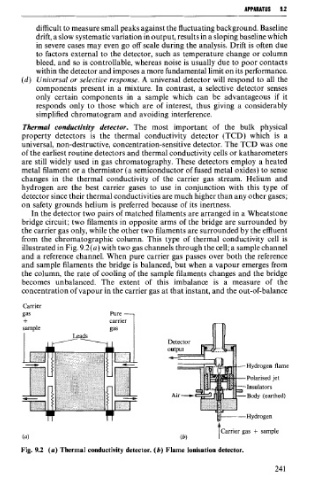
Thermal conductivity detector. The most important of the bulk physical
property detectors is the thermal conductivity detector (TCD) which is a
universal, non-destructive, concentration-sensitive detector. The TCD was one
of the earliest routine detectors and thermal conductivity cells or katharometers
are still widely used in gas chromatography. These detectors employ a heated
metal filament or a thermistor (a semiconductor of fused metal oxides) to sense
changes in the thermal conductivity of the carrier gas Stream. Helium and
hydrogen are the best carrier gases to use in conjunction with this type of
detector since their thermal conductivities are much higher than any other gases;
on safety grounds helium is preferred because of its inertness.
In the detector two pairs of matched filaments are arranged in a Wheatstone
bridge circuit; two filaments in opposite arms of the bridge are surrounded by
the carrier gas only, while the other two filaments are surrounded by the effluent
from the chromatographic column. This type of thermal conductivity ce11 is
illustrated in Fig. 9.2(a) with two gas channels through the cell; a sample channel
and a reference channel. When pure carrier gas passes over both the reference
and sample filaments the bridge is balanced, but when a vapour emerges from
the column, the rate of cooling of the sample filaments changes and the bridge
becomes unbalanced. The extent of this imbalance is a measure of the
concentration of vapour in the carrier gas at that instant, and the out-of-balance
Carrier
gas
+
sample
I
Leads
Hydrogen flame
. Polarised jet
-1nsulators
.Body (earthed)
t Carrier gas + sample
Fig. 9.2 (a) Thermal conductivity detector. (b) Flame ionisation detector.