Page 271 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 271
APPARATUS 9.2
A 8-ray source (commonly a foi1 containing 3H or 63Ni) is used to generate
'slow' electrons by ionisation of the carrier gas (nitrogen preferred) flowing
through the detector. These slow electrons migrate to the anode under a fixed
potential and give rise to a steady baseline current. When an electron-capturing
gas (i.e. eluate molecules) emerges from the column and reacts with an electron,
the net result is the replacement of an electron by a negative ion of much greater
mass with a corresponding reduction in current flow.
The response of the detector is clearly related to the electron affinity of the
eluate molecules being particularly sensitive to compounds containing halogens
and sulphur, anhydrides, conjugated carbonyls, nitrites, nitrates and organo-
metallic compounds. The ECD is the second most widely used ionisation detector
due to its high sensitivity to a wide range of compounds. It is, for example, used
in trace analysis of pesticides, herbicides, drugs, and other biologically active
compounds, and is of value in detecting ultratrace amounts of metals as their
chelate complexe^.^ '
Compared with the flame ionisation detector, however, the ECD is more
specialised and tends to be chosen for its selectivity which can simplify
chromatograms. The ECD requires careful attention to obtain reliable results.
Cleanliness is essential and the carrier gases must be very pure and dry. The
two most likely impurities in these gases are water and oxygen which are
sufficiently electronegative to produce a detector response and so give a noisy
baseline.
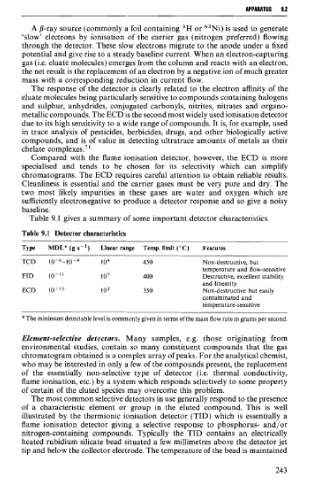
Table 9.1 gives a summary of some important detector characteristics.
Table 9.1 Detector characteristics
Type MDL* (g s-') Linear range Temp. limit (OC) Features
TCD 10-6-10-8 lo4 450 Non-destructive, but
temperature and flow-sensitive
FID IO-" 10' 400 Destructive, excellent stability
and linearity
ECD 10-1~ 102 350 Non-destructive but easily
contaminated and
temperature-sensitive
*The minimum detectable level is commonly given in terms of the mass flow rate in grams per second.
Element-selective detectors. Many samples, e.g. those originating from
environmental studies, contain so many constituent compounds that the gas
chromatogram obtained is a complex array of peaks. For the analytical chemist,
who may be interested in only a few of the compounds present, the replacement
of the essentially non-selective type of detector (i.e. thermal conductivity,
flame ionisation, etc.) by a system which responds selectively to some property
of certain of the eluted species may overcome this problem.
The most common selective detectors in use generally respond to the presence
of a characteristic element or group in the eluted compound. This is well
illustrated by the thermionic ionisation detector (TID) which is essentially a
flame ionisation detector giving a selective response to phosphorus- and/or
nitrogen-containing compounds. Typically the TID contains an electrically
heated rubidium silicate bead situated a few millimetres above the detector jet
tip and below the collector electrode. The temperature of the bead is maintained