Page 84 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 84
2 ANDAMENTAL THEORETICAL PRINCIPLES OF REACTIONS IN SOLUTION
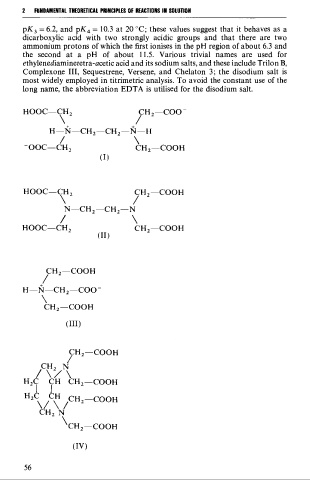
pK, = 6.2, and pK, = 10.3 at 20 OC; these values suggest that it behaves as a
dicarboxylic acid with two strongly acidic groups and that there are two
ammonium protons of which the first ionises in the pH region of about 6.3 and
the second at a pH of about 11.5. Various trivial names are used for
ethylenediaminetetra-acetic acid and its sodium salts, and these include Trilon B,
Complexone III, Sequestrene, Versene, and Chelaton 3; the disodium Salt is
most widely employed in titrimetric analysis. To avoid the constant use of the
long name, the abbreviation EDTA is utilised for the disodium salt.