Page 89 - Vogel's TEXTBOOK OF QUANTITATIVE CHEMICAL ANALYSIS
P. 89
ELECTRODE POTEWTIALS 2.28
If in equation (33), a,.+ is put equal to unity, E is equal to Ee. Ee is called
the standard electrode potential of the metal; both E and Ee are expressed in
volts.
In order to determine the potential difference between an electrode and a
solution, it is necessary to have another electrode and solution of accurately
known potential difference. The two electrodes can then be combined to form
a voltaic cell, the e.m.f. of which can be directly measured. The e.m.f. of the ce11
is the difference of the electrode potentials at zero current; the value of the
unknown potential can then be calculated. The primary reference electrode is
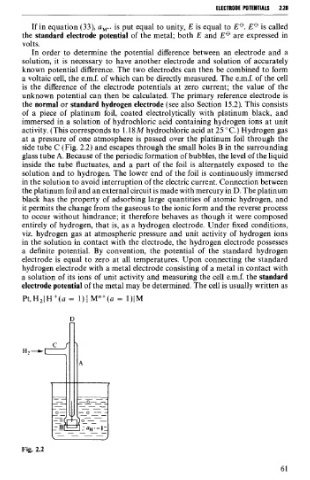
the normal or standard hydrogen electrode (see also Section 15.2). This consists
of a piece of platinum foil, coated electrolytically with platinum black, and
immersed in a solution of hydrochloric acid containing hydrogen ions at unit
activity. (This corresponds to 1.18 M hydrochloric acid at 25 OC.) Hydrogen gas
at a pressure of one atmosphere is passed over the platinum foil through the
side tube C (Fig. 2.2) and escapes through the small holes B in the surrounding
glass tube A. Because of the periodic formation of bubbles, the level of the liquid
inside the tube fluctuates, and a part of the foil is alternately exposed to the
solution and to hydrogen. The lower end of the foil is continuously immersed
in the solution to avoid interruption of the electric current. Connection between
the platinum foil and an external circuit is made with mercury in D. The platinum
black has the property of adsorbing large quantities of atomic hydrogen, and
it permits the change from the gaseous to the ionic form and the reverse process
to occur without hindrance; it therefore behaves as though it were composed
entirely of hydrogen, that is, as a hydrogen electrode. Under fixed conditions,
viz. hydrogen gas at atmospheric pressure and unit activity of hydrogen ions
in the solution in contact with the electrode, the hydrogen electrode possesses
a definite potential. By convention, the potential of the standard hydrogen
electrode is equal to zero at al1 temperatures. Upon connecting the standard
hydrogen electrode with a metal electrode consisting of a metal in contact with
a solution of its ions of unit activity and measuring the ce11 e.m.f. the standard
electrode potential of the metal may be determined. The ce11 is usually written as
Fig. 2.2