Page 297 - Materials Chemistry, Second Edition
P. 297
CAT3525_C09.qxd 2/8/2005 10:11 AM Page 268
268 Waste Management Practices: Municipal, Hazardous, and Industrial
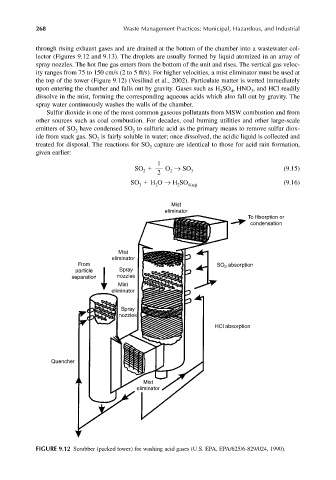
through rising exhaust gases and are drained at the bottom of the chamber into a wastewater col-
lector (Figures 9.12 and 9.13). The droplets are usually formed by liquid atomized in an array of
spray nozzles. The hot flue gas enters from the bottom of the unit and rises. The vertical gas veloc-
ity ranges from 75 to 150 cm/s (2 to 5 ft/s). For higher velocities, a mist eliminator must be used at
the top of the tower (Figure 9.12) (Vesilind et al., 2002). Particulate matter is wetted immediately
upon entering the chamber and falls out by gravity. Gases such as H SO , HNO , and HCl readily
4
3
2
dissolve in the mist, forming the corresponding aqueous acids which also fall out by gravity. The
spray water continuously washes the walls of the chamber.
Sulfur dioxide is one of the most common gaseous pollutants from MSW combustion and from
other sources such as coal combustion. For decades, coal burning utilities and other large-scale
emitters of SO have condensed SO to sulfuric acid as the primary means to remove sulfur diox-
2
2
ide from stack gas. SO is fairly soluble in water; once dissolved, the acidic liquid is collected and
2
treated for disposal. The reactions for SO capture are identical to those for acid rain formation,
2
given earlier:
1
SO O → SO (9.15)
2
2 2 3
SO H O → H SO 4(aq) (9.16)
3
2
2
Mist
eliminator
To filsorption or
condensation
Mist
eliminator
From SO 2 absorption
particle Spray
separation nozzles
Mist
eliminator
Spray
nozzles
HCl absorption
Quencher
Mist
eliminator
FIGURE 9.12 Scrubber (packed tower) for washing acid gases (U.S. EPA, EPA/625/6-829/024, 1990).