Page 326 - Water and wastewater engineering
P. 326
LIME–SODA SOFTENING 7-43
7-13. Using K sp , show why magnesium is removed as a hydroxide rather than a carbonate
in lime-soda softening.
7-14. Estimate the CO 2 concentration in mg/L as CO 2 and in mg/L as CaCO 3 for the water
analysis presented in Problem 7-5. Assume the water temperature was 4.4 C.
7-15. Estimate the CO 2 concentration in mg/L as CO 2 and in mg/L as CaCO 3 for the water
analysis presented in Problem 7-8. Assume the water temperature was 6 C.
7-16. If the pH of the MSU water (Problem 7-9) was 8.0 and the water temperature was
5 C, what is the estimated CO 2 concentration in mg/L as CO 2 and as mg/L as CaCO 3 ?
7-17. Estimate the CO 2 concentration in mg/L as CO 2 and in mg/L as CaCO 3 for the water
analysis presented in Problem 7-11. Assume the water temperature was 10 C. For the
estimate of the CO 2 concentration, ignore the carbonate alkalinity.
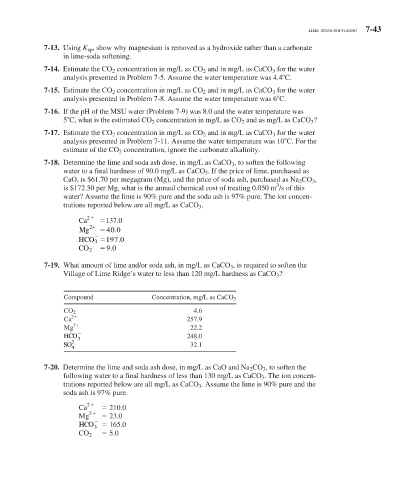
7-18. Determine the lime and soda ash dose, in mg/L as CaCO 3 , to soften the following
water to a final hardness of 90.0 mg/L as CaCO 3 . If the price of lime, purchased as
CaO, is $61.70 per megagram (Mg), and the price of soda ash, purchased as Na 2 CO 3 ,
3
is $172.50 per Mg, what is the annual chemical cost of treating 0.050 m /s of this
water? Assume the lime is 90% pure and the soda ash is 97% pure. The ion concen-
trations reported below are all mg/L as CaCO 3 .
2
Ca 137.0
Mg 2 40 0
.
HCO 3 197 0
.
90
.
CO 2
7-19. What amount of lime and/or soda ash, in mg/L as CaCO 3 , is required to soften the
Village of Lime Ridge’s water to less than 120 mg/L hardness as CaCO 3 ?
Compound Concentration, mg/L as CaCO 3
CO 2 4.6
2
Ca 257.9
2
Mg 22.2
HCO 3 248.0
2
SO 32.1
4
7-20. Determine the lime and soda ash dose, in mg/L as CaO and Na 2 CO 3 , to soften the
following water to a final hardness of less than 130 mg/L as CaCO 3 . The ion concen-
trations reported below are all mg/L as CaCO 3 . Assume the lime is 90% pure and the
soda ash is 97% pure.
2
Ca 210.0
2
Mg 23.0
HCO 165.0
3
CO 2 5.0