Page 327 - Water and wastewater engineering
P. 327
7-44 WATER AND WASTEWATER ENGINEERING
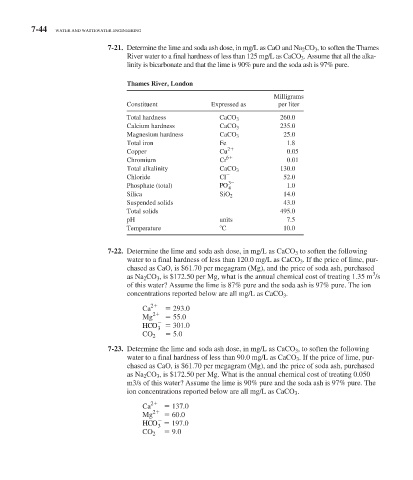
7-21. Determine the lime and soda ash dose, in mg/L as CaO and Na 2 CO 3 , to soften the Thames
River water to a final hardness of less than 125 mg/L as CaCO 3 . Assume that all the alka-
linity is bicarbonate and that the lime is 90% pure and the soda ash is 97% pure.
Thames River, London
Milligrams
Constituent Expressed as per liter
Total hardness CaCO 3 260.0
Calcium hardness CaCO 3 235.0
Magnesium hardness CaCO 3 25.0
Total iron Fe 1.8
2
Copper Cu 0.05
6
Chromium Cr 0.01
Total alkalinity CaCO 3 130.0
Chloride Cl 52.0
3
Phosphate (total) PO 4 1.0
Silica SiO 2 14.0
Suspended solids 43.0
Total solids 495.0
pH units 7.5
Temperature C 10.0
7-22. Determine the lime and soda ash dose, in mg/L as CaCO 3 to soften the following
water to a final hardness of less than 120.0 mg/L as CaCO 3 . If the price of lime, pur-
chased as CaO, is $61.70 per megagram (Mg), and the price of soda ash, purchased
3
as Na 2 CO 3 , is $172.50 per Mg, what is the annual chemical cost of treating 1.35 m /s
of this water? Assume the lime is 87% pure and the soda ash is 97% pure. The ion
concentrations reported below are all mg/L as CaCO 3 .
2
Ca 293.0
2
Mg 55.0
HCO 301.0
3
CO 2 5.0
7-23. Determine the lime and soda ash dose, in mg/L as CaCO 3 , to soften the following
water to a final hardness of less than 90.0 mg/L as CaCO 3 . If the price of lime, pur-
chased as CaO, is $61.70 per megagram (Mg), and the price of soda ash, purchased
as Na 2 CO 3 , is $172.50 per Mg. What is the annual chemical cost of treating 0.050
m3/s of this water? Assume the lime is 90% pure and the soda ash is 97% pure. The
ion concentrations reported below are all mg/L as CaCO 3 .
2
Ca 137.0
Mg 2 60.0
HCO 197.0
3
CO 2 9.0