Page 486 - Water and wastewater engineering
P. 486
MEMBRANE FILTRATION 12-3
Operating
pressures 700 200 70 35
(kPa)
Size 0.001 0.01 0.1 1.0 10 100 1,000
(micrometers)
Reverse
osmosis
Separation
Nano-filtration Microfiltration
processes
Ultrafiltration Conventional filtration
processes
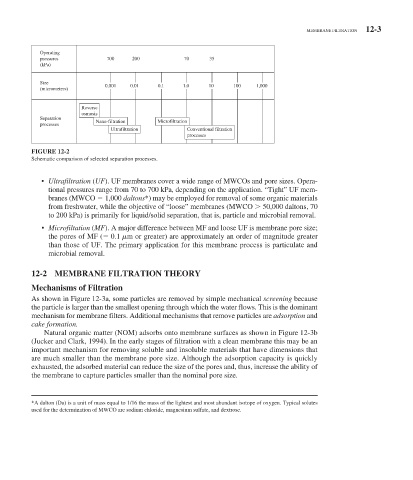
FIGURE 12-2
Schematic comparison of selected separation processes.
• Ultrafiltration ( UF ). UF membranes cover a wide range of MWCOs and pore sizes. Opera-
tional pressures range from 70 to 700 kPa, depending on the application. “Tight” UF mem-
branes (MWCO 1,000 daltons * ) may be employed for removal of some organic materials
from freshwater, while the objective of “loose” membranes (MWCO 50,000 daltons, 70
to 200 kPa) is primarily for liquid/solid separation, that is, particle and microbial removal.
• Microfiltation ( MF ). A major difference between MF and loose UF is membrane pore size;
the pores of MF ( 0.1 m or greater) are approximately an order of magnitude greater
than those of UF. The primary application for this membrane process is particulate and
microbial removal.
12-2 MEMBRANE FILTRATION THEORY
Mechanisms of Filtration
As shown in Figure 12-3a , some particles are removed by simple mechanical screening because
the particle is larger than the smallest opening through which the water flows. This is the dominant
mechanism for membrane filters. Additional mechanisms that remove particles are adsorption and
cake formation.
Natural organic matter (NOM) adsorbs onto membrane surfaces as shown in Figure 12-3b
(Jucker and Clark, 1994). In the early stages of filtration with a clean membrane this may be an
important mechanism for removing soluble and insoluble materials that have dimensions that
are much smaller than the membrane pore size. Although the adsorption capacity is quickly
exhausted, the adsorbed material can reduce the size of the pores and, thus, increase the ability of
the membrane to capture particles smaller than the nominal pore size.
* A dalton (Da) is a unit of mass equal to 1/16 the mass of the lightest and most abundant isotope of oxygen. Typical solutes
used for the determination of MWCO are sodium chloride, magnesium sulfate, and dextrose.