Page 204 - Well Control for Completions and Interventions
P. 204
Completion, Workover, and Intervention Fluids 197
the freezing point of seawater to 28.4 For 1.8 C. Similarly, salt dis-
solved in completion brine reduces the temperature at which freezing
occurs. If freezing does occur, water ice crystals begin to form in the
brine. Dissolving more salt both increases brine density and reduces the
freezing point down to the eutectic point. If the density is increased fur-
ther still, the crystallization temperature increases, but here, at higher den-
sities, cooling causes salt crystals to form.
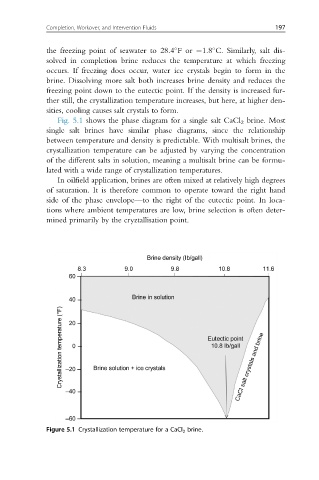
Fig. 5.1 shows the phase diagram for a single salt CaCl 2 brine. Most
single salt brines have similar phase diagrams, since the relationship
between temperature and density is predictable. With multisalt brines, the
crystallization temperature can be adjusted by varying the concentration
of the different salts in solution, meaning a multisalt brine can be formu-
lated with a wide range of crystallization temperatures.
In oilfield application, brines are often mixed at relatively high degrees
of saturation. It is therefore common to operate toward the right hand
side of the phase envelope—to the right of the eutectic point. In loca-
tions where ambient temperatures are low, brine selection is often deter-
mined primarily by the cryztallisation point.
Figure 5.1 Crystallization temperature for a CaCl 2 brine.