Page 206 - Well Control for Completions and Interventions
P. 206
Completion, Workover, and Intervention Fluids 199
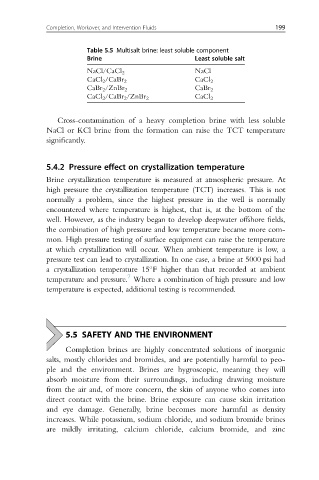
Table 5.5 Multisalt brine: least soluble component
Brine Least soluble salt
NaCl
NaCl/CaCl 2
CaCl 2 /CaBr 2 CaCl 2
CaBr 2 /ZnBr 2 CaBr 2
CaCl 2 /CaBr 2 /ZnBr 2 CaCl 2
Cross-contamination of a heavy completion brine with less soluble
NaCl or KCl brine from the formation can raise the TCT temperature
significantly.
5.4.2 Pressure effect on crystallization temperature
Brine crystallization temperature is measured at atmospheric pressure. At
high pressure the crystallization temperature (TCT) increases. This is not
normally a problem, since the highest pressure in the well is normally
encountered where temperature is highest, that is, at the bottom of the
well. However, as the industry began to develop deepwater offshore fields,
the combination of high pressure and low temperature became more com-
mon. High pressure testing of surface equipment can raise the temperature
at which crystallization will occur. When ambient temperature is low, a
pressure test can lead to crystallization. In one case, a brine at 5000 psi had
a crystallization temperature 15 F higher than that recorded at ambient
7
temperature and pressure. Where a combination of high pressure and low
temperature is expected, additional testing is recommended.
5.5 SAFETY AND THE ENVIRONMENT
Completion brines are highly concentrated solutions of inorganic
salts, mostly chlorides and bromides, and are potentially harmful to peo-
ple and the environment. Brines are hygroscopic, meaning they will
absorb moisture from their surroundings, including drawing moisture
from the air and, of more concern, the skin of anyone who comes into
direct contact with the brine. Brine exposure can cause skin irritation
and eye damage. Generally, brine becomes more harmful as density
increases. While potassium, sodium chloride, and sodium bromide brines
are mildly irritating, calcium chloride, calcium bromide, and zinc