Page 205 - Well Control for Completions and Interventions
P. 205
198 Well Control for Completions and Interventions
5.4.1 Determining crystallization temperature
Brine crystallization temperature is determined experimentally by repeat-
edly cooling and heating a brine sample.
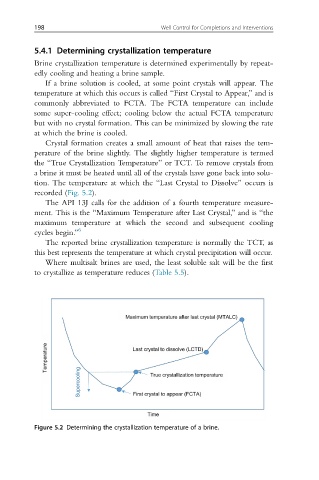
If a brine solution is cooled, at some point crystals will appear. The
temperature at which this occurs is called “First Crystal to Appear,” and is
commonly abbreviated to FCTA. The FCTA temperature can include
some super-cooling effect; cooling below the actual FCTA temperature
but with no crystal formation. This can be minimized by slowing the rate
at which the brine is cooled.
Crystal formation creates a small amount of heat that raises the tem-
perature of the brine slightly. The slightly higher temperature is termed
the “True Crystallization Temperature” or TCT. To remove crystals from
a brine it must be heated until all of the crystals have gone back into solu-
tion. The temperature at which the “Last Crystal to Dissolve” occurs is
recorded (Fig. 5.2).
The API 13J calls for the addition of a fourth temperature measure-
ment. This is the “Maximum Temperature after Last Crystal,” and is “the
maximum temperature at which the second and subsequent cooling
6
cycles begin.”
The reported brine crystallization temperature is normally the TCT, as
this best represents the temperature at which crystal precipitation will occur.
Where multisalt brines are used, the least soluble salt will be the first
to crystallize as temperature reduces (Table 5.5).
Figure 5.2 Determining the crystallization temperature of a brine.