Page 51 - Mechanical Behavior of Materials
P. 51
52 Chapter 2 Structure and Deformation in Materials
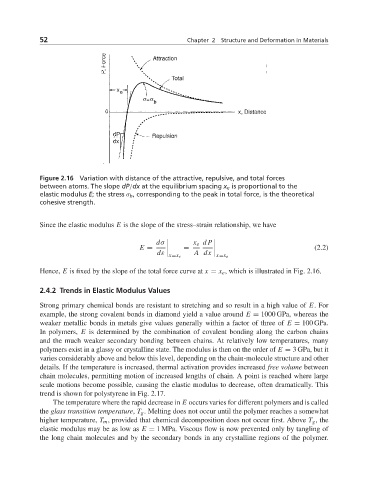
Figure 2.16 Variation with distance of the attractive, repulsive, and total forces
between atoms. The slope dP/dx at the equilibrium spacing x e is proportional to the
elastic modulus E; the stress σ b , corresponding to the peak in total force, is the theoretical
cohesive strength.
Since the elastic modulus E is the slope of the stress–strain relationship, we have
dσ x e dP
E = = (2.2)
dε A dx
x=x e x=x e
Hence, E is fixed by the slope of the total force curve at x = x e , which is illustrated in Fig. 2.16.
2.4.2 Trends in Elastic Modulus Values
Strong primary chemical bonds are resistant to stretching and so result in a high value of E.For
example, the strong covalent bonds in diamond yield a value around E = 1000 GPa, whereas the
weaker metallic bonds in metals give values generally within a factor of three of E = 100 GPa.
In polymers, E is determined by the combination of covalent bonding along the carbon chains
and the much weaker secondary bonding between chains. At relatively low temperatures, many
polymers exist in a glassy or crystalline state. The modulus is then on the order of E = 3GPa, but it
varies considerably above and below this level, depending on the chain-molecule structure and other
details. If the temperature is increased, thermal activation provides increased free volume between
chain molecules, permitting motion of increased lengths of chain. A point is reached where large
scale motions become possible, causing the elastic modulus to decrease, often dramatically. This
trend is shown for polystyrene in Fig. 2.17.
The temperature where the rapid decrease in E occurs varies for different polymers and is called
the glass transition temperature, T g . Melting does not occur until the polymer reaches a somewhat
higher temperature, T m , provided that chemical decomposition does not occur first. Above T g ,the
elastic modulus may be as low as E = 1 MPa. Viscous flow is now prevented only by tangling of
the long chain molecules and by the secondary bonds in any crystalline regions of the polymer.