Page 48 - Mechanical Behavior of Materials
P. 48
Section 2.3 Structure in Crystalline Materials 49
Figure 2.12 Crystal grain structure in a magnesium alloy containing 12 wt% lithium.
This cast metal was prepared in a high-frequency induction melting furnace under an
argon atmosphere. (Photo courtesy of Milo Kral, University of Canterbury, Christchurch,
New Zealand; used with permission.)
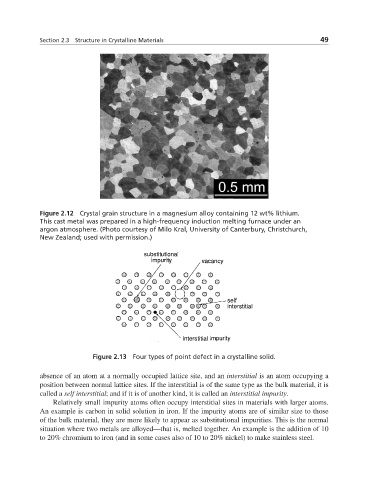
Figure 2.13 Four types of point defect in a crystalline solid.
absence of an atom at a normally occupied lattice site, and an interstitial is an atom occupying a
position between normal lattice sites. If the interstitial is of the same type as the bulk material, it is
called a self interstitial; and if it is of another kind, it is called an interstitial impurity.
Relatively small impurity atoms often occupy interstitial sites in materials with larger atoms.
An example is carbon in solid solution in iron. If the impurity atoms are of similar size to those
of the bulk material, they are more likely to appear as substitutional impurities. This is the normal
situation where two metals are alloyed—that is, melted together. An example is the addition of 10
to 20% chromium to iron (and in some cases also of 10 to 20% nickel) to make stainless steel.