Page 47 - Mechanical Behavior of Materials
P. 47
48 Chapter 2 Structure and Deformation in Materials
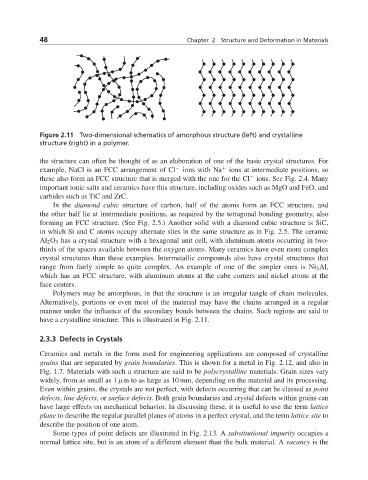
Figure 2.11 Two-dimensional schematics of amorphous structure (left) and crystalline
structure (right) in a polymer.
the structure can often be thought of as an elaboration of one of the basic crystal structures. For
example, NaCl is an FCC arrangement of Cl − ions with Na + ions at intermediate positions, so
−
these also form an FCC structure that is merged with the one for the Cl ions. See Fig. 2.4. Many
important ionic salts and ceramics have this structure, including oxides such as MgO and FeO, and
carbides such as TiC and ZrC.
In the diamond cubic structure of carbon, half of the atoms form an FCC structure, and
the other half lie at intermediate positions, as required by the tetragonal bonding geometry, also
forming an FCC structure. (See Fig. 2.5.) Another solid with a diamond cubic structure is SiC,
in which Si and C atoms occupy alternate sites in the same structure as in Fig. 2.5. The ceramic
Al 2 O 3 has a crystal structure with a hexagonal unit cell, with aluminum atoms occurring in two-
thirds of the spaces available between the oxygen atoms. Many ceramics have even more complex
crystal structures than these examples. Intermetallic compounds also have crystal structures that
range from fairly simple to quite complex. An example of one of the simpler ones is Ni 3 Al,
which has an FCC structure, with aluminum atoms at the cube corners and nickel atoms at the
face centers.
Polymers may be amorphous, in that the structure is an irregular tangle of chain molecules.
Alternatively, portions or even most of the material may have the chains arranged in a regular
manner under the influence of the secondary bonds between the chains. Such regions are said to
have a crystalline structure. This is illustrated in Fig. 2.11.
2.3.3 Defects in Crystals
Ceramics and metals in the form used for engineering applications are composed of crystalline
grains that are separated by grain boundaries. This is shown for a metal in Fig. 2.12, and also in
Fig. 1.7. Materials with such a structure are said to be polycrystalline materials. Grain sizes vary
widely, from as small as 1 μm to as large as 10 mm, depending on the material and its processing.
Even within grains, the crystals are not perfect, with defects occurring that can be classed as point
defects, line defects,or surface defects. Both grain boundaries and crystal defects within grains can
have large effects on mechanical behavior. In discussing these, it is useful to use the term lattice
plane to describe the regular parallel planes of atoms in a perfect crystal, and the term lattice site to
describe the position of one atom.
Some types of point defects are illustrated in Fig. 2.13. A substitutional impurity occupies a
normal lattice site, but is an atom of a different element than the bulk material. A vacancy is the