Page 46 - Mechanical Behavior of Materials
P. 46
Section 2.3 Structure in Crystalline Materials 47
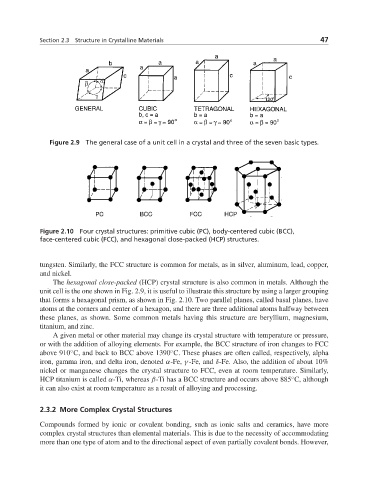
Figure 2.9 The general case of a unit cell in a crystal and three of the seven basic types.
Figure 2.10 Four crystal structures: primitive cubic (PC), body-centered cubic (BCC),
face-centered cubic (FCC), and hexagonal close-packed (HCP) structures.
tungsten. Similarly, the FCC structure is common for metals, as in silver, aluminum, lead, copper,
and nickel.
The hexagonal close-packed (HCP) crystal structure is also common in metals. Although the
unit cell is the one shown in Fig. 2.9, it is useful to illustrate this structure by using a larger grouping
that forms a hexagonal prism, as shown in Fig. 2.10. Two parallel planes, called basal planes, have
atoms at the corners and center of a hexagon, and there are three additional atoms halfway between
these planes, as shown. Some common metals having this structure are beryllium, magnesium,
titanium, and zinc.
A given metal or other material may change its crystal structure with temperature or pressure,
or with the addition of alloying elements. For example, the BCC structure of iron changes to FCC
above 910 C, and back to BCC above 1390 C. These phases are often called, respectively, alpha
◦
◦
iron, gamma iron, and delta iron, denoted α-Fe, γ -Fe, and δ-Fe. Also, the addition of about 10%
nickel or manganese changes the crystal structure to FCC, even at room temperature. Similarly,
HCP titanium is called α-Ti, whereas β-Ti has a BCC structure and occurs above 885 C, although
◦
it can also exist at room temperature as a result of alloying and processing.
2.3.2 More Complex Crystal Structures
Compounds formed by ionic or covalent bonding, such as ionic salts and ceramics, have more
complex crystal structures than elemental materials. This is due to the necessity of accommodating
more than one type of atom and to the directional aspect of even partially covalent bonds. However,