Page 41 - Mechanical Behavior of Materials
P. 41
42 Chapter 2 Structure and Deformation in Materials
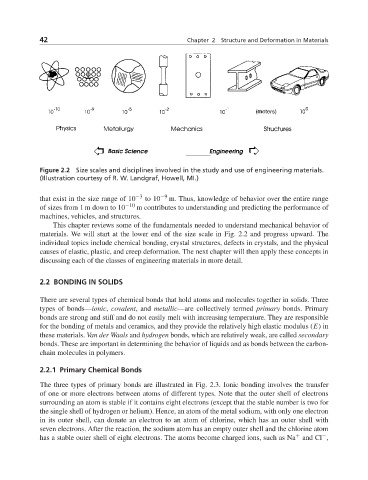
Figure 2.2 Size scales and disciplines involved in the study and use of engineering materials.
(Illustration courtesy of R. W. Landgraf, Howell, MI.)
that exist in the size range of 10 −3 to 10 −9 m. Thus, knowledge of behavior over the entire range
of sizes from 1 m down to 10 −10 m contributes to understanding and predicting the performance of
machines, vehicles, and structures.
This chapter reviews some of the fundamentals needed to understand mechanical behavior of
materials. We will start at the lower end of the size scale in Fig. 2.2 and progress upward. The
individual topics include chemical bonding, crystal structures, defects in crystals, and the physical
causes of elastic, plastic, and creep deformation. The next chapter will then apply these concepts in
discussing each of the classes of engineering materials in more detail.
2.2 BONDING IN SOLIDS
There are several types of chemical bonds that hold atoms and molecules together in solids. Three
types of bonds—ionic, covalent, and metallic—are collectively termed primary bonds. Primary
bonds are strong and stiff and do not easily melt with increasing temperature. They are responsible
for the bonding of metals and ceramics, and they provide the relatively high elastic modulus (E)in
these materials. Van der Waals and hydrogen bonds, which are relatively weak, are called secondary
bonds. These are important in determining the behavior of liquids and as bonds between the carbon-
chain molecules in polymers.
2.2.1 Primary Chemical Bonds
The three types of primary bonds are illustrated in Fig. 2.3. Ionic bonding involves the transfer
of one or more electrons between atoms of different types. Note that the outer shell of electrons
surrounding an atom is stable if it contains eight electrons (except that the stable number is two for
the single shell of hydrogen or helium). Hence, an atom of the metal sodium, with only one electron
in its outer shell, can donate an electron to an atom of chlorine, which has an outer shell with
seven electrons. After the reaction, the sodium atom has an empty outer shell and the chlorine atom
−
+
has a stable outer shell of eight electrons. The atoms become charged ions, such as Na and Cl ,