Page 44 - Mechanical Behavior of Materials
P. 44
Section 2.2 Bonding in Solids 45
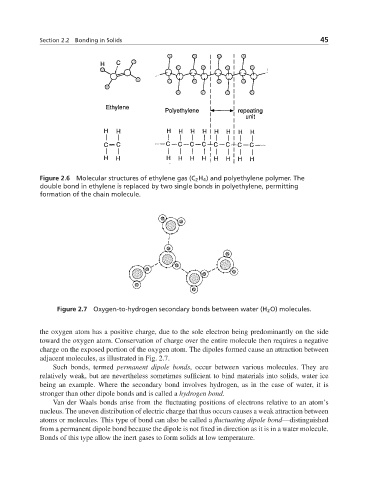
Figure 2.6 Molecular structures of ethylene gas (C 2 H 4 ) and polyethylene polymer. The
double bond in ethylene is replaced by two single bonds in polyethylene, permitting
formation of the chain molecule.
Figure 2.7 Oxygen-to-hydrogen secondary bonds between water (H 2 O) molecules.
the oxygen atom has a positive charge, due to the sole electron being predominantly on the side
toward the oxygen atom. Conservation of charge over the entire molecule then requires a negative
charge on the exposed portion of the oxygen atom. The dipoles formed cause an attraction between
adjacent molecules, as illustrated in Fig. 2.7.
Such bonds, termed permanent dipole bonds, occur between various molecules. They are
relatively weak, but are nevertheless sometimes sufficient to bind materials into solids, water ice
being an example. Where the secondary bond involves hydrogen, as in the case of water, it is
stronger than other dipole bonds and is called a hydrogen bond.
Van der Waals bonds arise from the fluctuating positions of electrons relative to an atom’s
nucleus. The uneven distribution of electric charge that thus occurs causes a weak attraction between
atoms or molecules. This type of bond can also be called a fluctuating dipole bond—distinguished
from a permanent dipole bond because the dipole is not fixed in direction as it is in a water molecule.
Bonds of this type allow the inert gases to form solids at low temperature.