Page 139 - Materials Chemistry, Second Edition
P. 139
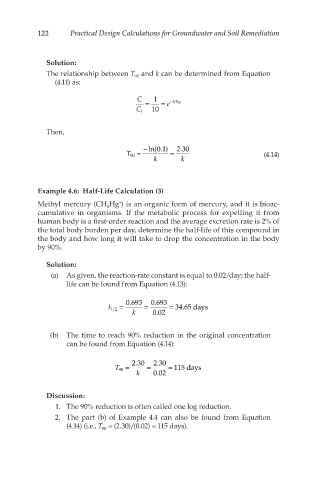
122 Practical Design Calculations for Groundwater and Soil Remediation
Solution:
The relationship between T and k can be determined from Equation
90
(4.11) as:
C 1
= = e − kT 90
C i 10
Then,
− ln(0.1) 2.30
T 90 = = (4.14)
k k
Example 4.6: Half-Life Calculation (3)
+
Methyl mercury (CH Hg ) is an organic form of mercury, and it is bioac-
3
cumulative in organisms. If the metabolic process for expelling it from
human body is a first-order reaction and the average excretion rate is 2% of
the total body burden per day, determine the half-life of this compound in
the body and how long it will take to drop the concentration in the body
by 90%.
Solution:
(a) As given, the reaction-rate constant is equal to 0.02/day; the half-
life can be found from Equation (4.13):
0.693 0.693
t 1/2 = = = 34.65 days
k 0.02
(b) The time to reach 90% reduction in the original concentration
can be found from Equation (4.14):
2.30 2.30
T 90 = = = 115 days
k 0.02
Discussion:
1. The 90% reduction is often called one log reduction.
2. The part (b) of Example 4.4 can also be found from Equation
(4.14) (i.e., T = (2.30)/(0.02) = 115 days).
90