Page 143 - Materials Chemistry, Second Edition
P. 143
126 Practical Design Calculations for Groundwater and Soil Remediation
Strategy:
It requires a two-step approach to solve this problem. The first is to
determine the rate constant using the given information. Then, use
this obtained k value to estimate the residence time for other conver-
sions. The given information did not tell us the order of the reaction.
We assume it is a first-order reaction, but this should be confirmed
with additional test data.
Solution:
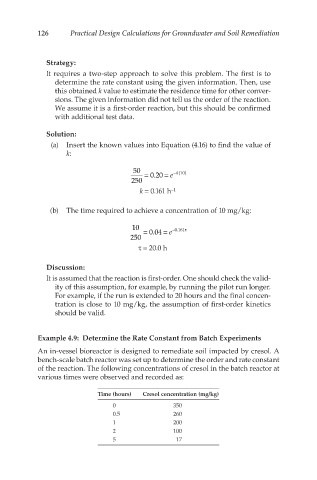
(a) Insert the known values into Equation (4.16) to find the value of
k:
50 = 0.20 = e − k(10)
250
k = 0.161 h −1
(b) The time required to achieve a concentration of 10 mg/kg:
10 = 0.04 = e − 0.161τ
250
τ = 20.0 h
Discussion:
It is assumed that the reaction is first-order. One should check the valid-
ity of this assumption, for example, by running the pilot run longer.
For example, if the run is extended to 20 hours and the final concen-
tration is close to 10 mg/kg, the assumption of first-order kinetics
should be valid.
Example 4.9: Determine the Rate Constant from Batch Experiments
An in-vessel bioreactor is designed to remediate soil impacted by cresol. A
bench-scale batch reactor was set up to determine the order and rate constant
of the reaction. The following concentrations of cresol in the batch reactor at
various times were observed and recorded as:
Time (hours) Cresol concentration (mg/kg)
0 350
0.5 260
1 200
2 100
5 17