Page 265 -
P. 265
OXIDATION AND DISINFECTION 10.11
Oxidizing agents, or oxidants, used in water treatment include chlorine, chlorine diox-
ide, permanganate, oxygen, and ozone. The appropriate oxidant for achieving a specific
water quality objective depends on a number of factors, including raw water quality, spe-
cific contaminants, and local chemical and power costs. For critical applications, the de-
signer should insist on bench- or pilot-scale evaluations of treatment alternatives to select
the best approach and determine appropriate design criteria.
Iron and Manganese Removal
Iron and manganese have secondary MCLs (SMCLs) of 0.3 and 0.05 mg/L, respectively.
These SMCLs have been considered as safe limits to avoid the staining of plumbing fix-
tures and laundry, but experience shows that lower levels are desired to avoid difficul-
ties. Targets of < 0.1 mg/L iron and < 0.02 mg/L manganese should be normal water
quality goals.
Dissolved iron and manganese are normally in the reduced state (Fe II and Mn II) and
can be removed by oxidizing to Fe III and Mn IV, where they will precipitate as Fe(OH)3
and Mn(OH)2. Precipitates are subsequently removed in sedimentation and/or filtration
steps. Several oxidants are available for this process, namely, chlorine, chlorine dioxide,
ozone, and potassium permanganate. They are also removed through conventional lime
softening treatment. The stoichiometric requirements for each oxidant are given in Tables
10.6 and 10.7. Most oxidants also react with organics in the water, so some testing is nor-
mally required to determine the appropriate dose.
Small well water systems with excessive levels of iron and manganese often apply an
oxidant, provide a period of detention for the reaction to take place, and then remove
the precipitated iron and manganese with a pressure filter. Figure 10.5 illustrates such a
system.
Chlorine is an effective oxidant for iron, and oxidation to Fe(OH)3 proceeds rapidly.
It is not as effective for manganese removal, however, at normal pH conditions. Chlorine
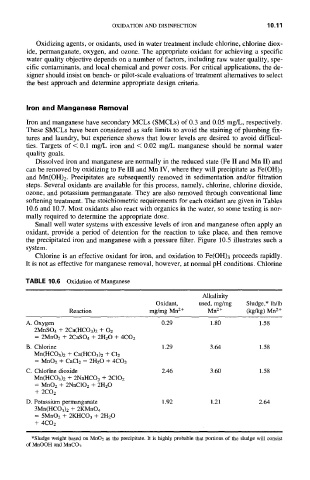
TABLE 10.6 Oxidation of Manganese
Alkalinity
Oxidant, used, mg/mg Sludge,* lb/lb
Reaction mg/mg Mn 2+ Mn 2+ (kg/kg) Mn 2+
A. Oxygen 0.29 1.80 1.58
2MnSO4 + 2Ca(HCO3) 2 + 02
= 2MnO2 + 2CASO4 + 2H20 + 4CO2
B. Chlorine 1.29 3.64 1.58
Mn(HCO3)2 + Ca(HCO3)2 + C12
= MnO2 + CaCI2 - 2H2 O + 4CO2
C. Chlorine dioxide 2.46 3.60 1.58
Mn(HCO3)2 + 2NaHCO3 + 2C102
= MnO2 + 2NaCIO2 + 2H20
+ 2CO2
D. Potassium permanganate 1.92 1.21 2.64
3Mn(HCO3)2 + 2KMnO4
= 5MNO2 + 2KHCO3 + 2H20
+ 4CO2
*Sludge weight based on MnO2 as the precipitate. It is highly probable that portions of the sludge will consist
of MnOOH and MnCO~.