Page 289 -
P. 289
Next Page
OXIDATION AND DISINFECTION 10.33
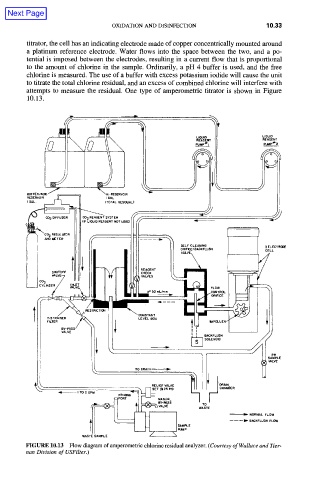
titrator, the cell has an indicating electrode made of copper concentrically mounted around
a platinum reference electrode. Water flows into the space between the two, and a po-
tential is imposed between the electrodes, resulting in a current flow that is proportional
to the amount of chlorine in the sample. Ordinarily, a pH 4 buffer is used, and the free
chlorine is measured. The use of a buffer with excess potassium iodide will cause the unit
to titrate the total chlorine residual, and an excess of combined chlorine will interfere with
attempts to measure the residual. One type of amperometric titrator is shown in Figure
10.13.
I
LIQUIO
LIOUIO
REAG NT REAGENT
puMp~'* 2
BUFFER/ACID
I GAL
(TOTAL RESIOUAL )
J
Go~ ........ II II co2 .... E.~ ..... fKF~
$EL'CLE .... I I 3 ..... '~
O~!E!CS~SACKFLUS" CELL
[ i BACKFLU$H
~ SOLENOID
1=.
SAMPLE
, VALVE
k TO ORA,~
CHAMBER
q IT
IlL NORMAL FLOW
------ID- BACKFLUSH FLOW
FIGURE 10.13 Flow diagram of amperometric chlorine residual analyzer. (Courtesy of Wallace and Tier-
nan Division of USFilter.)