Page 294 -
P. 294
10.38 CHAPTER TEN
Chlorine
dioxide
solution
to process Ejector
Ejector
water
supply
Reaction zone
m
Feed rate
~ meters
I
Chlorite
] Chlorine gas
supply supply
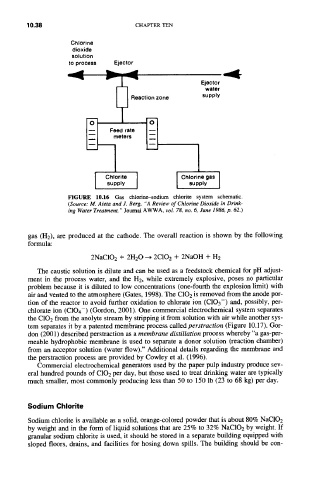
FIGURE 10.16 Gas chlorine-sodium chlorite system schematic.
(Source: M. Aieta and J. Berg, "A Review of Chlorine Dioxide in Drink-
ing Water Treatment," Journal AWWA, vol. 78, no. 6, June 1986, p. 62.)
gas (H2), are produced at the cathode. The overall reaction is shown by the following
formula:
2NaCIO2 + 2H20 ~ 2C102 + 2NaOH + H2
The caustic solution is dilute and can be used as a feedstock chemical for pH adjust-
ment in the process water, and the H2, while extremely explosive, poses no particular
problem because it is diluted to low concentrations (one-fourth the explosion limit) with
air and vented to the atmosphere (Gates, 1998). The C102 is removed from the anode por-
tion of the reactor to avoid further oxidation to chlorate ion (CIO3-) and, possibly, per-
chlorate ion (C104-) (Gordon, 2001). One commercial electrochemical system separates
the C102 from the anolyte stream by stripping it from solution with air while another sys-
tem separates it by a patented membrane process called perstraction (Figure 10.17). Gor-
don (2001) described perstraction as a membrane distillation process whereby "a gas-per-
meable bydrophobic membrane is used to separate a donor solution (reaction chamber)
from an acceptor solution (water flow)." Additional details regarding the membrane and
the perstraction process are provided by Cowley et al. (1996).
Commercial electrochemical generators used by the paper pulp industry produce sev-
eral hundred pounds of C102 per day, but those used to treat drinking water are typically
much smaller, most commonly producing less than 50 to 150 lb (23 to 68 kg) per day.
Sodium Chlorite
Sodium chlorite is available as a solid, orange-colored powder that is about 80% NaC102
by weight and in the form of liquid solutions that are 25% to 32% NaC102 by weight. If
granular sodium chlorite is used, it should be stored in a separate building equipped with
sloped floors, drains, and facilities for hosing down spills. The building should be con-