Page 308 -
P. 308
10.50 CHAPTER TEN
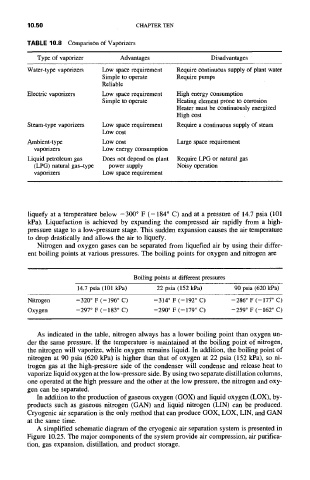
TABLE 10.8 Comparison of Vaporizers
Type of vaporizer Advantages Disadvantages
Water-type vaporizers Low space requirement Require continuous supply of plant water
Simple to operate Require pumps
Reliable
Electric vaporizers Low space requirement High energy consumption
Heating element prone to corrosion
Simple to operate
Heater must be continuously energized
High cost
Steam-type vaporizers Low space requirement Require a continuous supply of steam
Low cost
Large space requirement
Ambient-type Low cost
vaporizers Low energy consumption
Liquid petroleum gas Does not depend on plant Require LPG or natural gas
(LPG) natural gas-type power supply Noisy operation
vaporizers Low space requirement
liquefy at a temperature below -300 ° F (-184 ° C) and at a pressure of 14.7 psia (101
kPa). Liquefaction is achieved by expanding the compressed air rapidly from a high-
pressure stage to a low-pressure stage. This sudden expansion causes the air temperature
to drop drastically and allows the air to liquefy.
Nitrogen and oxygen gases can be separated from liquefied air by using their differ-
ent boiling points at various pressures. The boiling points for oxygen and nitrogen are
Boiling points at different pressures
14.7 psia (101 kPa) 22 psia (152 kPa) 90 psia (620 kPa)
Nitrogen -320 ° F (-196 ° C) -314 ° F (-192 ° C) -286 ° F (-177 ° C)
Oxygen -297 ° F (-183 ° C) -290 ° F (-179 ° C) -259 ° F (-162 ° C)
As indicated in the table, nitrogen always has a lower boiling point than oxygen un-
der the same pressure. If the temperature is maintained at the boiling point of nitrogen,
the nitrogen will vaporize, while oxygen remains liquid. In addition, the boiling point of
nitrogen at 90 psia (620 kPa) is higher than that of oxygen at 22 psia (152 kPa), so ni-
trogen gas at the high-pressure side of the condenser will condense and release heat to
vaporize liquid oxygen at the low-pressure side. By using two separate distillation columns,
one operated at the high pressure and the other at the low pressure, the nitrogen and oxy-
gen can be separated.
In addition to the production of gaseous oxygen (GOX) and liquid oxygen (LOX), by-
products such as gaseous nitrogen (GAN) and liquid nitrogen (LIN) can be produced.
Cryogenic air separation is the only method that can produce GOX, LOX, LIN, and GAN
at the same time.
A simplified schematic diagram of the cryogenic air separation system is presented in
Figure 10.25. The major components of the system provide air compression, air purifica-
tion, gas expansion, distillation, and product storage.