Page 181 - A Comprehensive Guide to Solar Energy Systems
P. 181
Chapter 9 • Crystalline Silicon Solar Cell and Module Technology 183
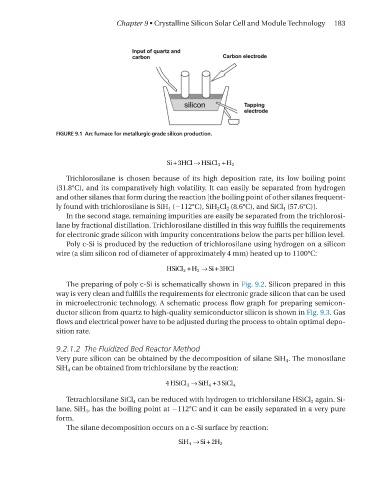
FIGURE 9.1 Arc furnace for metallurgic-grade silicon production.
Si 3HCl → HSiCl 3 + H 2
+
Si+3hCl→hSiCl 3 +h 2
Trichlorosilane is chosen because of its high deposition rate, its low boiling point
(31.8°C), and its comparatively high volatility. It can easily be separated from hydrogen
and other silanes that form during the reaction [the boiling point of other silanes frequent-
ly found with trichlorosilane is Sih 4 (−112°C), Sih 2 Cl 2 (8.6°C), and SiCl 4 (57.6°C)].
In the second stage, remaining impurities are easily be separated from the trichlorosi-
lane by fractional distillation. Trichlorosilane distilled in this way fulfills the requirements
for electronic grade silicon with impurity concentrations below the parts per billion level.
Poly c-Si is produced by the reduction of trichlorosilane using hydrogen on a silicon
wire (a slim silicon rod of diameter of approximately 4 mm) heated up to 1100°C:
HSiCl 3 + H 2 → Si 3HCl hSiCl 3 +h 2 →Si+3hCl
+
The preparing of poly c-Si is schematically shown in Fig. 9.2. Silicon prepared in this
way is very clean and fulfills the requirements for electronic grade silicon that can be used
in microelectronic technology. A schematic process flow graph for preparing semicon-
ductor silicon from quartz to high-quality semiconductor silicon is shown in Fig. 9.3. Gas
flows and electrical power have to be adjusted during the process to obtain optimal depo-
sition rate.
9.2.1.2 The Fluidized Bed Reactor Method
Very pure silicon can be obtained by the decomposition of silane Sih 4 . The monosilane
Sih 4 can be obtained from trichlorsilane by the reaction:
4HSiCl 3 → SiH 4 + 3SiCl 4
4 hSiCl 3 →Sih 4 +3 SiCl 4
Tetrachlorsilane SiCl 4 can be reduced with hydrogen to trichlorsilane hSiCl 3 again. Si-
lane, Sih 4 , has the boiling point at −112°C and it can be easily separated in a very pure
form.
The silane decomposition occurs on a c-Si surface by reaction:
+
SiH 4 → Si 2H 2
Sih 4 →Si+2h 2