Page 184 - A Comprehensive Guide to Solar Energy Systems
P. 184
186 A COmPrehenSIVe GUIDe TO SOlAr enerGy SySTemS
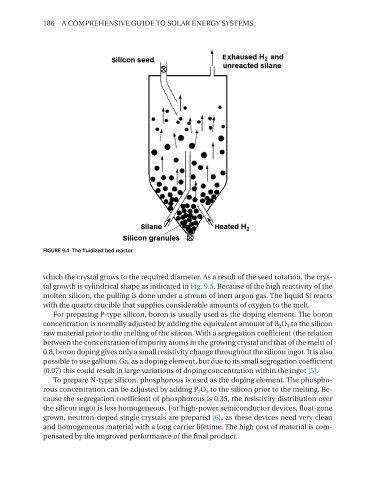
FIGURE 9.4 The fluidized bed reactor.
which the crystal grows to the required diameter. As a result of the seed rotation, the crys-
tal growth is cylindrical shape as indicated in Fig. 9.5. Because of the high reactivity of the
molten silicon, the pulling is done under a stream of inert argon gas. The liquid Si reacts
with the quartz crucible that supplies considerable amounts of oxygen to the melt.
For preparing P-type silicon, boron is usually used as the doping element. The boron
concentration is normally adjusted by adding the equivalent amount of B 2 O 3 to the silicon
raw material prior to the melting of the silicon. With a segregation coefficient (the relation
between the concentration of impurity atoms in the growing crystal and that of the melt) of
0.8, boron doping gives only a small resistivity change throughout the silicon ingot. It is also
possible to use gallium, Ga, as a doping element, but due to its small segregation coefficient
(0.07) this could result in large variations of doping concentration within the ingot [5].
To prepare n-type silicon, phosphorous is used as the doping element. The phospho-
rous concentration can be adjusted by adding P 2 O 5 to the silicon prior to the melting. Be-
cause the segregation coefficient of phosphorous is 0.35, the resistivity distribution over
the silicon ingot is less homogeneous. For high-power semiconductor devices, float-zone
grown, neutron-doped single crystals are prepared [6], as these devices need very clean
and homogeneous material with a long carrier lifetime. The high cost of material is com-
pensated by the improved performance of the final product.