Page 98 - Hybrid Enhanced Oil Recovery Using Smart Waterflooding
P. 98
90 Hybrid Enhanced Oil Recovery using Smart Waterflooding
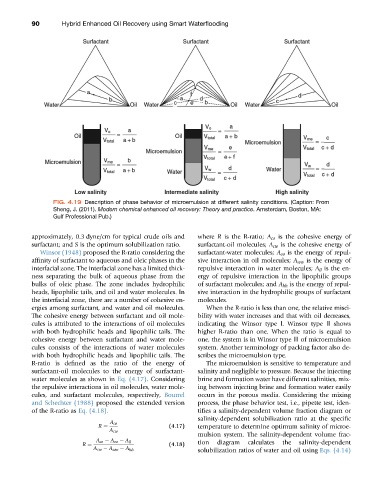
FIG. 4.19 Description of phase behavior of microemulsion at different salinity conditions. (Caption: From
Sheng, J. (2011). Modern chemical enhanced oil recovery: Theory and practice. Amsterdam, Boston, MA:
Gulf Professional Pub.)
approximately, 0.3 dyne/cm for typical crude oils and where R is the R-ratio; A co is the cohesive energy of
surfactant; and S is the optimum solubilization ratio. surfactant-oil molecules; A cw is the cohesive energy of
Winsor (1948) proposed the R-ratio considering the surfactant-water molecules; A oo is the energy of repul-
affinity of surfactant to aqueous and oleic phases in the sive interaction in oil molecules; A ww is the energy of
interfacial zone. The interfacial zone has a limited thick- repulsive interaction in water molecules; A ll is the en-
ness separating the bulk of aqueous phase from the ergy of repulsive interaction in the lipophilic groups
bulks of oleic phase. The zone includes hydrophilic of surfactant molecules; and A hh is the energy of repul-
heads, lipophilic tails, and oil and water molecules. In sive interaction in the hydrophilic groups of surfactant
the interfacial zone, there are a number of cohesive en- molecules.
ergies among surfactant, and water and oil molecules. When the R-ratio is less than one, the relative misci-
The cohesive energy between surfactant and oil mole- bility with water increases and that with oil decreases,
cules is attributed to the interactions of oil molecules indicating the Winsor type Ⅰ. Winsor type Ⅱ shows
with both hydrophilic heads and lipophilic tails. The higher R-ratio than one. When the ratio is equal to
cohesive energy between surfactant and water mole- one, the system is in Winsor type Ⅲ of microemulsion
cules consists of the interactions of water molecules system. Another terminology of packing factor also de-
with both hydrophilic heads and lipophilic tails. The scribes the microemulsion type.
R-ratio is defined as the ratio of the energy of The microemulsion is sensitive to temperature and
surfactant-oil molecules to the energy of surfactant- salinity and negligible to pressure. Because the injecting
water molecules as shown in Eq. (4.17). Considering brine and formation water have different salinities, mix-
the repulsive interactions in oil molecules, water mole- ing between injecting brine and formation water easily
cules, and surfactant molecules, respectively, Bourrel occurs in the porous media. Considering the mixing
and Schechter (1988) proposed the extended version process, the phase behavior test, i.e., pipette test, iden-
of the R-ratio as Eq. (4.18). tifies a salinity-dependent volume fraction diagram or
salinity-dependent solubilization ratio at the specific
A co
R ¼ (4.17) temperature to determine optimum salinity of microe-
A cw
mulsion system. The salinity-dependent volume frac-
A co A oo A ll tion diagram calculates the salinity-dependent
R ¼ (4.18)
solubilization ratios of water and oil using Eqs. (4.14)
A cw A ww A hh