Page 103 - Hybrid Enhanced Oil Recovery Using Smart Waterflooding
P. 103
CHAPTER 4 Hybrid Chemical EOR Using Low-Salinity and Smart Waterflood 95
The experimental studies (Hosseinzade Khanamiri,
1
Baltzersen Enge, et al., 2016; Hosseinzade Khanamiri,
0.8 Nourani, et al., 2016) continued to investigate factors
affecting the EOR potential of hybrid LSSF process in
0.6 LSS Berea sandstone. The studies adjusted the concentra-
C/Co tion of cations of Na ,Ca 2þ ,and Mg 2þ as well as
þ
0.4 OSS
salinity in the composition of brine. The high-
salinity in situ brine has 31,061 ppm TDS. The
0.2
low-salinity water as the 10-times-diluted in situ brine
0 is prepared. There are modified versions of in situ brine
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 and low-salinity water. The modified versions of in situ
Injected volume [PV]
and low-salinity brines are prepared by reducing the
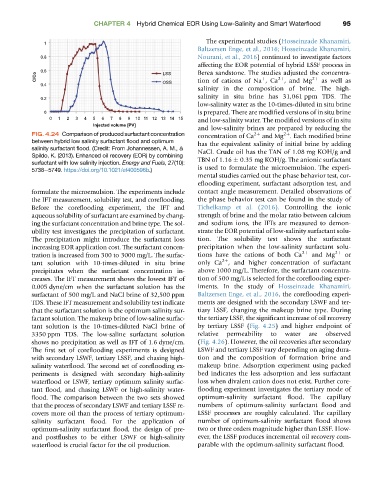
FIG. 4.24 Comparison of produced surfactant concentration concentration of Ca 2þ and Mg 2þ . Each modified brine
between hybrid low salinity surfactant flood and optimum has the equivalent salinity of initial brine by adding
salinity surfactant flood. (Credit: From Johannessen, A. M., & NaCl. Crude oil has the TAN of 1.08 mg KOH/g and
Spildo, K. (2013). Enhanced oil recovery (EOR) by combining
surfactant with low salinity injection. Energy and Fuels, 27(10): TBN of 1.16 0.35 mg KOH/g. The anionic surfactant
5738e5749. https://doi.org/10.1021/ef400596b.) is used to formulate the microemulsion. The experi-
mental studies carried out the phase behavior test, cor-
eflooding experiment, surfactant adsorption test, and
formulate the microemulsion. The experiments include contact angle measurement. Detailed observations of
the IFT measurement, solubility test, and coreflooding. the phase behavior test can be found in the study of
Before the coreflooding experiment, the IFT and Tichelkamp et al. (2016). Controlling the ionic
aqueous solubility of surfactant are examined by chang- strength of brine and the molar ratio between calcium
ing the surfactant concentration and brine type. The sol- and sodium ions, the IFTs are measured to demon-
ubility test investigates the precipitation of surfactant. strate the EOR potential of low-salinity surfactant solu-
The precipitation might introduce the surfactant loss tion. The solubility test shows the surfactant
increasing EOR application cost. The surfactant concen- precipitation when the low-salinity surfactant solu-
tration is increased from 300 to 3000 mg/L. The surfac- tions have the cations of both Ca 2þ and Mg 2þ or
tant solution with 10-times-diluted in situ brine only Ca 2þ , and higher concentration of surfactant
precipitates when the surfactant concentration in- above 1000 mg/L. Therefore, the surfactant concentra-
creases. The IFT measurement shows the lowest IFT of tion of 500 mg/L is selected for the coreflooding exper-
0.005 dyne/cm when the surfactant solution has the iments. In the study of Hosseinzade Khanamiri,
surfactant of 500 mg/L and NaCl brine of 32,500 ppm Baltzersen Enge, et al., 2016,the coreflooding experi-
TDS. These IFT measurement and solubility test indicate ments are designed with the secondary LSWF and ter-
that the surfactant solution is the optimum salinity sur- tiary LSSF, changing the makeup brine type. During
factant solution. The makeup brine of low-saline surfac- the tertiary LSSF, the significant increase of oil recovery
tant solution is the 10-times-diluted NaCl brine of by tertiary LSSF (Fig. 4.25) and higher endpoint of
3350 ppm TDS. The low-saline surfactant solution relative permeability to water are observed
shows no precipitation as well as IFT of 1.6 dyne/cm. (Fig. 4.26). However, the oil recoveries after secondary
The first set of coreflooding experiments is designed LSWF and tertiary LSSF vary depending on aging dura-
with secondary LSWF, tertiary LSSF, and chasing high- tion and the composition of formation brine and
salinity waterflood. The second set of coreflooding ex- makeup brine. Adsorption experiment using packed
periments is designed with secondary high-salinity bed indicates the less adsorption and less surfactant
waterflood or LSWF, tertiary optimum salinity surfac- loss when divalent cation does not exist. Further core-
tant flood, and chasing LSWF or high-salinity water- flooding experiment investigates the tertiary mode of
flood. The comparison between the two sets showed optimum-salinity surfactant flood. The capillary
that the process of secondary LSWF and tertiary LSSF re- numbers of optimum-salinity surfactant flood and
covers more oil than the process of tertiary optimum- LSSF processes are roughly calculated. The capillary
salinity surfactant flood. For the application of number of optimum-salinity surfactant flood shows
optimum-salinity surfactant flood, the design of pre- two or three orders magnitude higher than LSSF. How-
and postflushes to be either LSWF or high-salinity ever, the LSSF produces incremental oil recovery com-
waterflood is crucial factor for the oil production. parable with the optimum-salinity surfactant flood.