Page 83 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 83
-
Electrochemistry I: Galvanic Cells 67
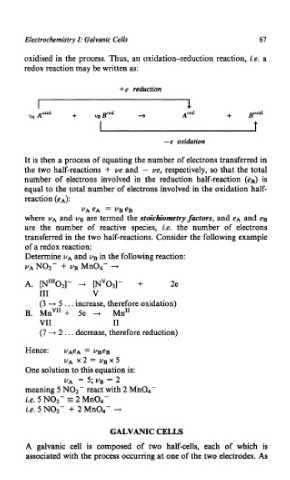
oxidised in the process. Thus, an oxidation-reduction reaction, i.e. a
redox reaction may be written as:
,
+e reduction
Aoxid. + + Ared. + pxid.
VA vBB'cd*
-e oxidation
It is then a process of equating the number of electrons transferred in
the two half-reactions + ve and - ue, respectively, so that the total
number of electrons involved in the reduction half-reaction (eB) is
equal to the total number of electrons involved in the oxidation half-
reaction (eA):
VAeA = UBeB
where UA and UB are termed the stoichiornetryfactors, and eA and eB
are the number of reactive species, i.e. the number of electrons
transferred in the two half-reactions. Consider the following example
of a redox reaction:
Determine UA and UB in the following reaction:
VANOZ- + UB Mn04- +
+
A. [""02]- + mv03]- 2e
I11 V
(3 --.) 5 . . . increase, therefore oxidation)
B. MnV" + 5e + Mn"
VII I1
(7 + 2 . . . decrease, therefore reduction)
Hence: UAeA = vBeB
UA x2 = vBX5
One solution to this equation is:
UA = 5; ug = 2
meaning 5 NO2- react with 2 Mn04-
i.e. 5 N02- s 2 Mn04-
i.e. 5 N02- + 2 Mn04- 4
GALVANIC CELLS
A galvanic cell is composed of two half-cells, each of which is
associated with the process occurring at one of the two electrodes. As