Page 84 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 84
68 Chapter 6
in a redox reaction in inorganic chemistry, the oxidation half-reaction
is not divorced from the reduction half-reaction, but both collectively
act as a couple.
+ e reduction = electrode A
vA Aoxid* + VgBICd. -+ AWd. + Exid.
I t
- e oxidation E electrode B
Similarly, it is impossible to isolate one half-cell from the other, nor
can the electrode potential of one half-cell be measured without
reference to the second half-cell.
In any electrochemical cell (both galvanic and electrolytic cells), the
electrode at which reduction takes place is called the cathode, and the
electrode at which oxidation takes place is called the anode. A useful
way of remembering this is the mnemonic ‘CROA’, i.e. cathode-
reduction anode-xidation.
Types of Electrodes Used in Galvanic Cells
There are four basic types of electrodes used in galvanic cells:
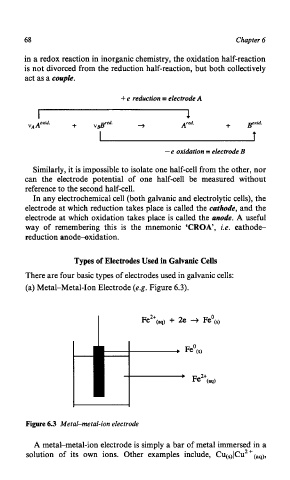
(a) Metal-Metal-Ion Electrode (e.g. Figure 6.3).
I I
Figure 6.3 Metal-metal-ion electrode
A metal-metal-ion electrode is simply a bar of metal immersed in a
solution of its own ions. Other examples include, CU&U~+(~~),