Page 80 - [B._MURPHY,_C._MURPHY,_B._HATHAWAY]_A_working_meth
P. 80
64 Chapter 6
Figure 6.1. For a conservative system, potential energy + kinetic
energy = constant.
In Chapters 6 and 7, the interconversion between two of these forms
of energy, electrical and chemical energy will be considered. Electro-
chemistry is concerned with the chemical changes produced by an
electric current and the production of electricity by a chemical
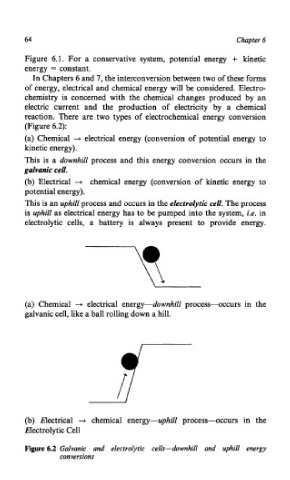
reaction. There are two types of electrochemical energy conversion
(Figure 6.2):
(a) Chemical + electrical energy (conversion of potential energy to
kinetic energy).
This is a downhill process and this energy conversion occurs in the
galvanic cell.
(b) Electrical + chemical energy (conversion of kinetic energy to
potential energy).
This is an uphill process and occurs in the electrolytic cell. The process
is uphill as electrical energy has to be pumped into the system, i.e. in
electrolytic cells, a battery is always present to provide energy.
(a) Chemical + electrical energy-downhill processvccurs in the
galvanic cell, like a ball rolling down a hill.
(b) Electrical + chemical energy-uphill process--occurs in the
Electrolytic Cell
Figure 6.2 Galvanic and electrolytic cells-downhill and uphill energy
conversions