Page 108 - Adsorbents fundamentals and applications
P. 108
ADSORPTION FROM SOLUTION AND EFFECTS OF SURFACE FUNCTIONALITIES 93
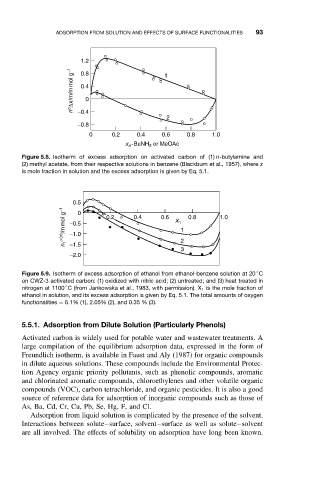
1.2 1
n o ∆x/m/m mol g −1 0.4
0.8
0
−0.4
2
−0.8
0 0.2 0.4 0.6 0.8 1.0
x -BuNH or MeOAc
n
2
Figure 5.8. Isotherm of excess adsorption on activated carbon of (1) n-butylamine and
(2) methyl acetate, from their respective solutions in benzene (Blackburn et al., 1957), where x
is mole fraction in solution and the excess adsorption is given by Eq. 5.1.
0.5
n 1 s(n) /mmol g −1 −0.5 0.2 0.4 0.6 X 1 1 0.8 1.0
0
−1.0
2
−1.5
3
−2.0
◦
Figure 5.9. Isotherm of excess adsorption of ethanol from ethanol-benzene solution at 20 C
on CWZ-3 activated carbon: (1) oxidized with nitric acid; (2) untreated; and (3) heat treated in
◦
nitrogen at 1100 C (from Jankowska et al., 1983, with permission). X 1 is the mole fraction of
ethanol in solution, and its excess adsorption is given by Eq. 5.1. The total amounts of oxygen
functionalities = 6.1% (1), 2.05% (2), and 0.35 % (3).
5.5.1. Adsorption from Dilute Solution (Particularly Phenols)
Activated carbon is widely used for potable water and wastewater treatments. A
large compilation of the equilibrium adsorption data, expressed in the form of
Freundlich isotherm, is available in Faust and Aly (1987) for organic compounds
in dilute aqueous solutions. These compounds include the Environmental Protec-
tion Agency organic priority pollutants, such as phenolic compounds, aromatic
and chlorinated aromatic compounds, chloroethylenes and other volatile organic
compounds (VOC), carbon tetrachloride, and organic pesticides. It is also a good
source of reference data for adsorption of inorganic compounds such as those of
As,Ba, Cd,Cr, Cu,Pb, Se, Hg, F, andCl.
Adsorption from liquid solution is complicated by the presence of the solvent.
Interactions between solute–surface, solvent–surface as well as solute–solvent
are all involved. The effects of solubility on adsorption have long been known.